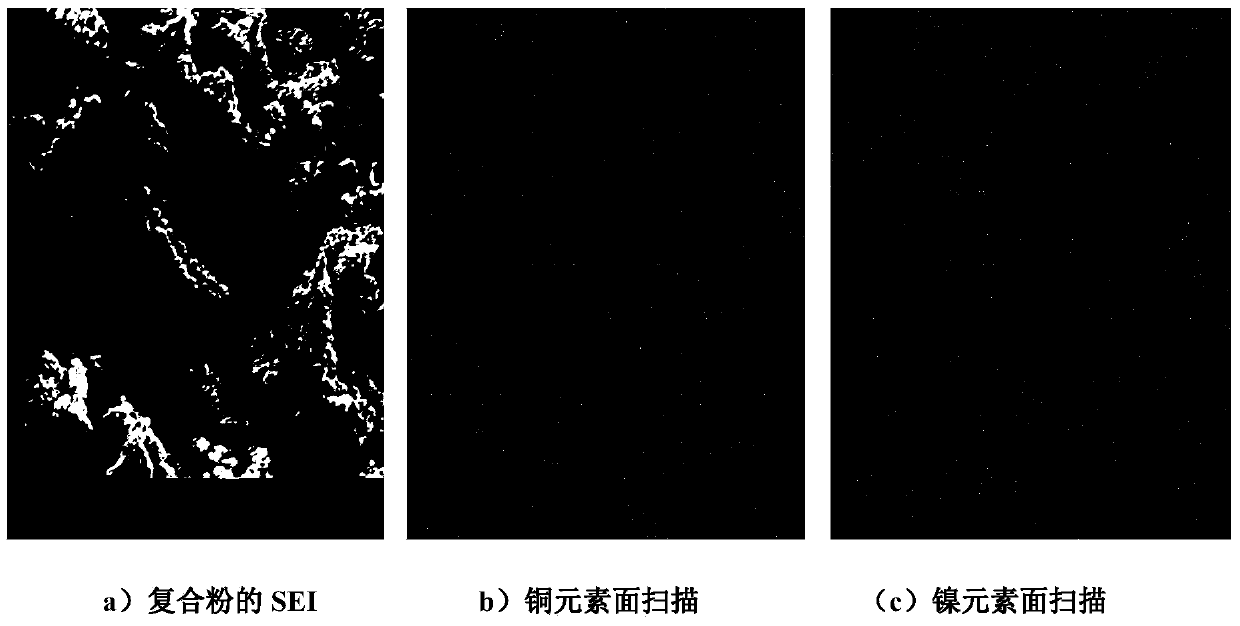
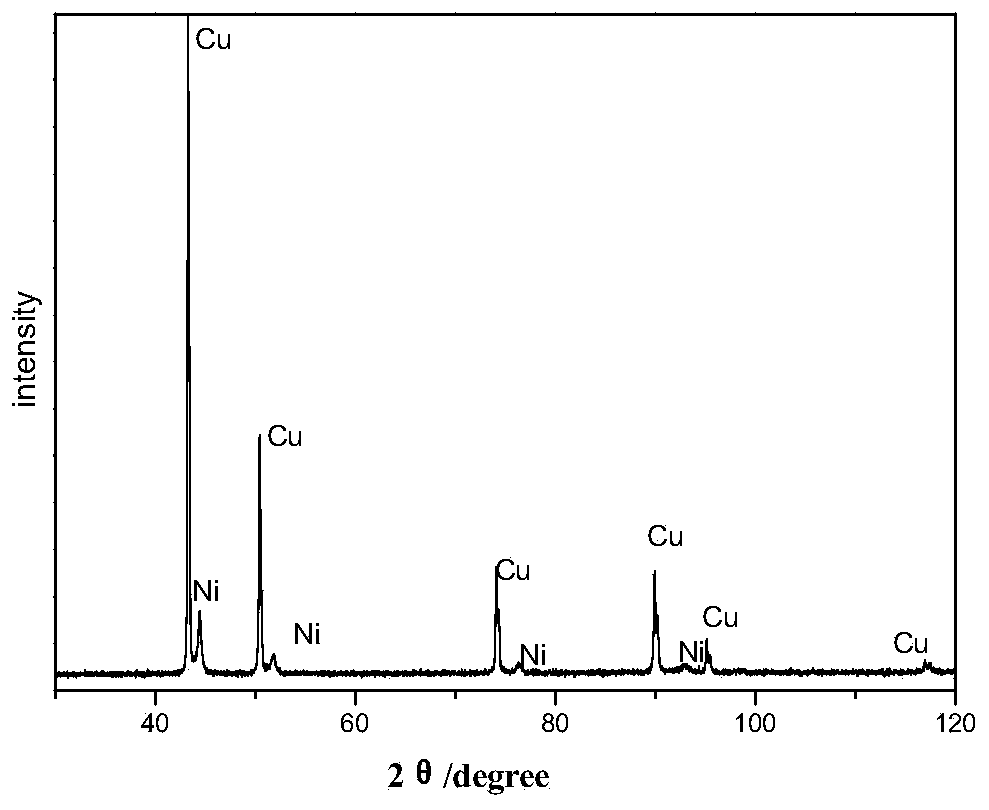
Method for preparing nickel-coated copper composite powder in laboratory through electroplating
A composite powder, nickel-coated copper technology, applied in the direction of the electrode, etc., can solve the problems of high cost, low nickel deposition rate, complex process, etc., and achieve the effect of simple preparation and pure nickel-copper interface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] Example 1: Nickel-coated copper powder was prepared using a nickel mesh as a cathode.
[0035] 1) The electronic balance accurately weighs the required chemical reagents: NiSO 4 .6H 2 O: 320g, NiCl 2 .6H 2 O: 50g, H 2 BO 3 : 45g, SDS: 0.1g, accurate to 0.01g;
[0036] 2) First, measure half the volume of the required plating solution, add quantitatively weighed nickel sulfate and nickel chloride, and stir vigorously until completely dissolved;
[0037] 3) Add an appropriate amount of water to another container to dissolve boric acid, and after the boric acid is completely dissolved, add it to the solution 2) under stirring;
[0038] 4) Use hydrochloric acid to adjust the pH value of the plating solution to a specified value of 4; pour the solution into a volumetric flask of a certain volume, then add quantitatively weighed SDS, adjust the volume of the plating solution to the specified value of 1L with distilled water, and then pour it into a beaker ;
[0039] 5...
Embodiment 2
[0042] Example 2: Using a nickel plate as a cathode to prepare nickel-coated copper powder.
[0043] 1) The electronic balance accurately weighs the required chemical reagents: NiSO 4 .6H 2 O: 320g, NiCl 2 .6H 2 O: 50g, H 2 BO 3 : 45g, SDS: 0.1g, accurate to 0.01g;
[0044] 2) First, measure half the volume of the required plating solution, add quantitatively weighed nickel sulfate and nickel chloride, and stir vigorously until completely dissolved;
[0045] 3) Add an appropriate amount of water to another container to dissolve boric acid, and after the boric acid is completely dissolved, add it to the solution 2) under stirring;
[0046] 4) Use hydrochloric acid to adjust the pH value of the plating solution to a specified value of 4; pour the solution into a volumetric flask of a certain volume, then add quantitatively weighed SDS, adjust the volume of the plating solution to the specified value of 1L with distilled water, and then pour it into a beaker ;
[0047] 5)...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap