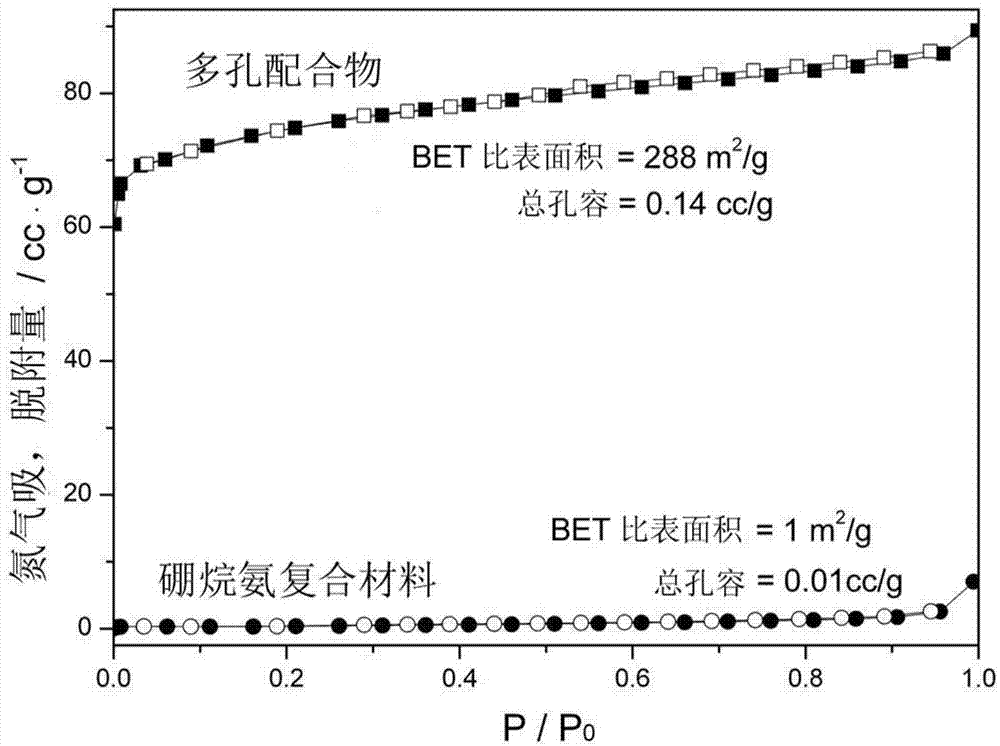
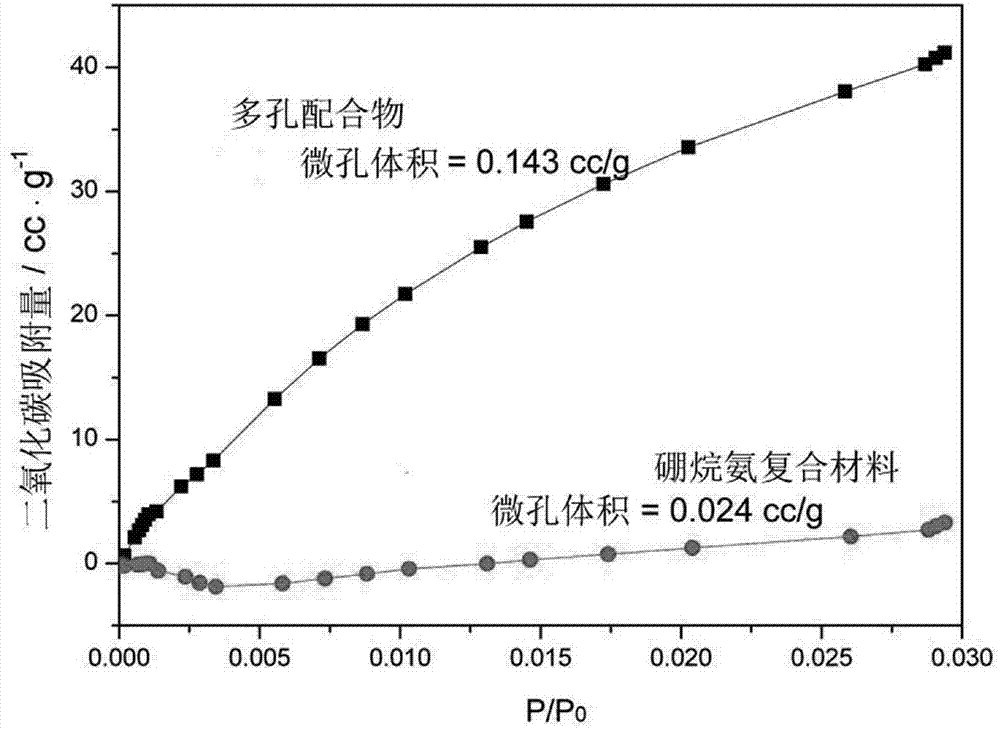
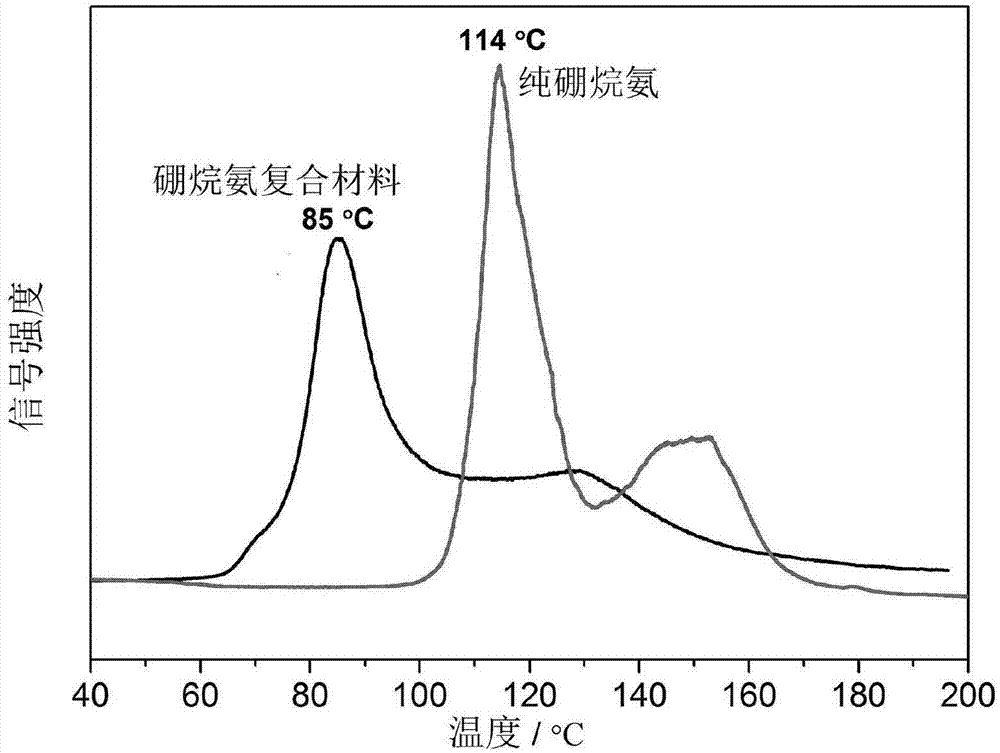
Ammonia borane composite hydrogen storage material and preparation method thereof
A hydrogen storage material, borane ammonia technology, applied in the direction of hydrogen production, etc., can solve problems that need to be improved, solve technical obstacles, reduce thermal decomposition and hydrogen release temperature, and achieve good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A borane ammonia composite hydrogen storage material, the preparation steps are as follows:
[0040] Step 1, in a glass beaker, 3.6g Zn(NO 3 ) 2 ·6H 2 O and 0.66g of terephthalic acid were placed in 196mL of N, N'-dimethylformamide, stirred until completely dissolved, and then 4mL of H 2 O, stir well. Zn(NO 3 ) 2 ·6H 2 The concentration of O is 0.012mol / L.
[0041] Step 2, transfer the mixed solution obtained in step 1 to a high temperature resistant glass reaction bottle with a cover, and place it in a constant temperature drying oven at 80° C. for 12 hours.
[0042] In step 3, the suspension obtained in step 2 was filtered, and the solid part was evacuated at 200° C. for 24 hours with a vacuum degree of 1 Pa to obtain a hollow porous coordination polymer.
[0043] Step 4, dissolve 0.05 g of borane ammonia in 10 mL of anhydrous methanol in a glove box at room temperature, and the glove box is a high-purity argon atmosphere.
[0044] Step 5, take 0.2 g of the p...
Embodiment 2
[0049] A borane ammonia composite hydrogen storage material, the preparation steps are as follows:
[0050] Step 1, in a glass beaker, 7.2g Zn(NO 3 ) 2 ·6H 2 O and 1.32g of terephthalic acid were placed in 196mL of N, N'-dimethylformamide, stirred until completely dissolved, and then 4mL of H 2 O, stir well. Zn(NO 3 ) 2 ·6H 2 The concentration of O is 0.024mol / L.
[0051] Step 2, transfer the mixed solution obtained in step 1 to a high temperature resistant glass reaction bottle with a cover, and place it in a constant temperature drying oven at 100° C. for 12 hours.
[0052] In step 3, the suspension obtained in step 2 was filtered, and the solid part was evacuated at 200° C. for 12 hours with a degree of vacuum of 0.1 Pa to obtain a hollow porous coordination polymer.
[0053] Step 4, dissolve 0.1 g of borane ammonia in 10 mL of anhydrous tetrahydrofuran in a glove box at room temperature, and the glove box is a high-purity argon atmosphere.
[0054] Step 5, take 0....
Embodiment 3
[0056] A borane ammonia composite hydrogen storage material, the preparation steps are as follows:
[0057] Step 1, in a glass beaker, 3.6g Zn(NO 3 ) 2 ·6H 2 O and 0.66g of terephthalic acid were placed in 196mL of N, N'-dimethylformamide, stirred until completely dissolved, and then 4mL of H 2 O, stir well. Zn(NO 3 ) 2 ·6H 2 The concentration of O is 0.012mol / L.
[0058] Step 2, transfer the mixed solution obtained in step 1 to a high temperature resistant glass reaction bottle with a cover, and place it in a constant temperature drying oven at 90° C. for 12 hours.
[0059] In step 3, the suspension obtained in step 2 was filtered, and the solid part was evacuated at 200° C. for 12 hours with a degree of vacuum of 0.1 Pa to obtain a hollow porous coordination polymer.
[0060] Step 4, dissolve 0.05 g of borane ammonia in 10 mL of anhydrous tetrahydrofuran in a glove box at room temperature, and the glove box is a high-purity argon atmosphere.
[0061] Step 5, take 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com