Traditional Chinese medicine composition for treating asthma as well as preparation method and application thereof
The technology of a composition and a traditional Chinese medicine is applied in the field of traditional Chinese medicine composition for treating asthma and its preparation field, which can solve the problems of insufficient attention to both the root cause and anti-inflammatory and allergy treatment, insufficient variety of Chinese patent medicines, weak synthesis force, etc., and achieves improved efficacy , Simple to use, reducing the effect of recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
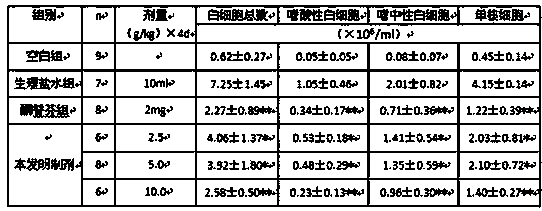
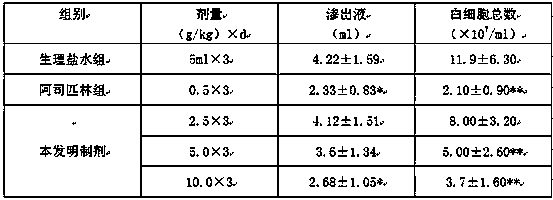
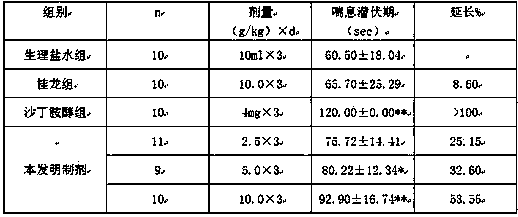
Image
Examples
Embodiment 1
[0181] a) Extract volatile oil from 333g tangerine peel, 483g bupleurum and 333g perilla leaves by steam distillation, and inclusion of volatile oil with 10 times the amount of β-cyclodextrin to obtain the first inclusion;
[0182] b) 600g ephedra, 600g ginseng, 1200g pangolin, and 333g magnolia were soaked in 70% ethanol for 24 hours, then leaked, collected the leaked liquid, recovered ethanol under reduced pressure, and concentrated to 25°C. The density is 1.08-1.12, and the extract II is obtained;
[0183] c) Combine the above Qiwei medicinal residues with 600g licorice, add 10 times the amount of water to decoct twice, add 333g bitter almonds after boiling, for 2 hours each time, filter the decoction, combine the filtrates, and concentrate to 80 °C when the relative density is 1.05-1.10, to obtain the extract III;
[0184] d) Combine the extract II and extract III and spray dry (inlet air temperature 160°C, outlet air temperature 90°C) to obtain dry powder, which is dried...
Embodiment 2
[0186] a) Extract volatile oil from 333g tangerine peel, 483g bupleurum and 333g perilla leaves by steam distillation, and inclusion of volatile oil with 10 times the amount of β-cyclodextrin to obtain the first inclusion;
[0187] b) 600g ephedra, 600g ginseng, 1200g pangolin, and 333g magnolia were soaked in 70% ethanol for 24 hours, then leaked, collected the leaked liquid, recovered ethanol under reduced pressure, and concentrated to 25°C. The density is 1.08-1.12, and the extract II is obtained;
[0188] c) Combine the above Qiwei medicinal residues with 600g licorice, add 10 times the amount of water to decoct twice, add 333g bitter almonds after boiling, for 2 hours each time, filter the decoction, combine the filtrates, and concentrate to 80 °C when the relative density is 1.05-1.10, to obtain the extract III;
[0189] d) Combine the extract II and extract III, and spray dry (inlet air temperature 160°C, outlet air temperature 90°C) to obtain dry powder, add the first...
Embodiment 3
[0191] a) Extract volatile oil from 333g tangerine peel, 483g bupleurum and 333g perilla leaves by steam distillation, and inclusion of volatile oil with 10 times the amount of β-cyclodextrin to obtain the first inclusion;
[0192] b) 600g ephedra, 600g ginseng, 1200g pangolin, and 333g magnolia were soaked in 70% ethanol for 24 hours, then leaked, collected the leaked liquid, recovered ethanol under reduced pressure, and concentrated to 25°C. The density is 1.08-1.12, and the extract II is obtained;
[0193] c) Combine the above Qiwei medicinal residues with 600g licorice, add 10 times the amount of water to decoct twice, add 333g bitter almonds after boiling, for 2 hours each time, filter the decoction, combine the filtrates, and concentrate to 80 °C when the relative density is 1.05-1.10, to obtain the extract III;
[0194] d) Combine extract II and extract III, spray dry (inlet air temperature 160°C, outlet air temperature 90°C) to obtain dry powder, add the first clathra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com