Preparation method for alpha-amyl cinnamic aldehyde dimethyl acetal
A technology of cinnamaldehyde dimethyl acetal and pentyl cinnamaldehyde, which is applied in the field of fine chemical synthesis, can solve problems such as complicated processing procedures, affecting product quality, and deep product color, and achieves the advantages of improved purity, easy purification, and long-lasting aroma Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
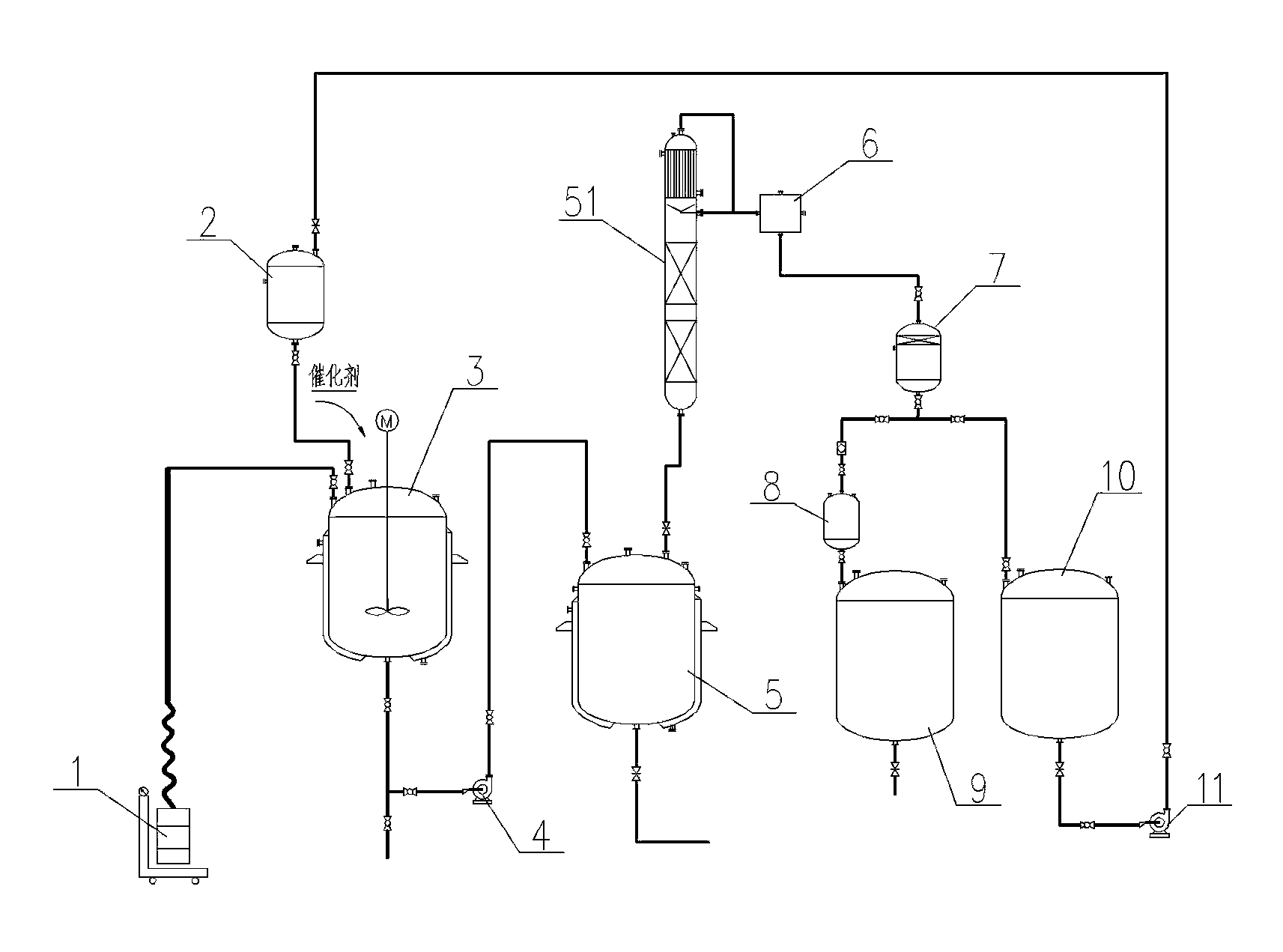
[0020] a) Use vacuum suction device 1 to add 200kg of α-amylcinnamaldehyde and 10kg of water-carrying cyclohexane into the 500L enamel reaction kettle 3. After the feeding is completed, replace the vacuum with nitrogen gas and add from the high tank 2 Methanol 80kg, add catalyst silica gel loaded zirconium sulfate 10kg from the hand hole of the reaction kettle, start the mixer and mix evenly;
[0021] b) Open the steam valve, heat up the enamel reaction kettle 3, and control the reaction temperature to 85~90°C;
[0022] c) After a reaction time of 4 hours, take a sample for gas chromatography detection, and when the content of α-amylcinnamaldehyde is less than 2%, cool down to terminate the reaction;
[0023] d) After the reaction is over, add 75kg of water to wash with water, and after standing for 2 hours, separate the lower layer of water through the observation of the mirror;
[0024] e) Transfer the upper oil layer of the reaction product in the enamel reaction kettle 3 ...
Embodiment 2
[0027] a) Add 240kg of α-amylcinnamic aldehyde and 12kg of water-containing cyclohexane to the 500L enamel reaction kettle by vacuum suction. After the feeding is completed, replace the vacuum with nitrogen, and add 100kg of methanol from the high-level tank , add 12 kg of ferric sulfate loaded on catalyst silica gel from the hand hole of the reaction kettle, start the mixer and mix evenly;
[0028] b) Open the steam valve, heat up the reactor, and control the reaction temperature to 85~90°C;
[0029] c) After a reaction time of 4 hours, take a sample for gas chromatography detection, and when the content of α-amylcinnamaldehyde is less than 2%, cool down to terminate the reaction;
[0030] d) After the reaction, add 80 kg of water, wash with water, let stand for 2 hours, and separate the lower layer of water through the observation mirror;
[0031] e) Transfer the upper oil layer of the reaction product in step d) to the fractionation still, raise the temperature of the stil...
Embodiment 3
[0034] a) Add 1,200kg of α-amylcinnamaldehyde and 60kg of water-containing cyclohexane to the 2500L enamel reaction kettle by vacuum suction. After the feeding is completed, replace the vacuum with nitrogen, and add 500kg of methanol from the high-level tank , add 55 kg of ferric sulfate loaded on catalyst silica gel from the hand hole of the reaction kettle, start the mixer and mix evenly;
[0035] b) Open the steam valve, heat up the reactor, and control the reaction temperature to 85~90°C;
[0036] c) After a reaction time of 4 hours, take a sample for gas chromatography detection, and when the content of α-amylcinnamaldehyde is less than 2%, cool down to terminate the reaction;
[0037] d) After the reaction is over, add 300kg of water to wash with water, and after standing for 2 hours, separate the lower layer of water through observation through the mirror;
[0038] e) Transfer the upper oil layer of the reaction product in step d) to the fractionating still, raise the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com