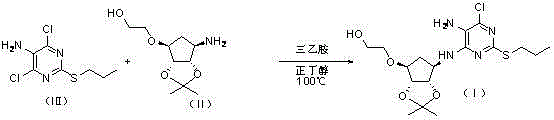A kind of preparation method of ticagrelor intermediate
A technology of ticagrelor and intermediates, applied in the field of medicine, can solve the problems affecting compound preparation efficiency, solvent and raw material side reactions, low conversion rate of raw materials, etc., and achieve the effect of simple operation, good product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
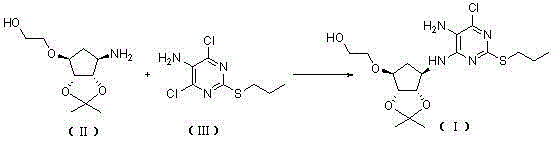
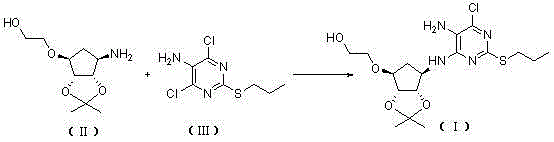
[0065] Example 1, 2-[((3aR,4S,6R,6aS)-6-[[5-amino-6-chloro-2-(propylthio)pyrimidin-4-yl]amino]-2,2- Preparation of Dimethyltetrahydro-3aH-Cyclopenta[d][1,3]dioxol-4-yl)oxy]ethanol (Compound Ⅰ)
[0066]
[0067] Take a 250mL reaction bottle, add 4,6-dichloro-2-(propylthio)pyrimidin-5-amine (16.1g, 68mmol), 2-[[(3aR,4S,6R,6aS)-6-amino- 2,2-Dimethyltetrahydro-3aH-cyclopentadieno[d][1,3]-dioxol-4-yl]oxy]-1-ethanol-dibenzoyl-L - Tartrate (17.3g, 68mmol), N,N-diisopropylethylamine (34.3g, 340mmol) and n-butanol (49mL). The resulting reaction mixture was heated to 90°C under airtight and kept at this temperature for 35h. It was then cooled to 30°C. The solvent was evaporated. Isopropyl acetate and water were added and the phases were separated. The aqueous phase was extracted with isopropyl acetate, and the organic phases were combined and washed with water. Dry over anhydrous magnesium sulfate. filter. The solvent was evaporated to obtain a reddish-brown oil. After addin...
Embodiment 2
[0068] Example 2, 2-[((3aR,4S,6R,6aS)-6-[[5-amino-6-chloro-2-(propylthio)pyrimidin-4-yl]amino]-2,2- Preparation of Dimethyltetrahydro-3aH-Cyclopenta[d][1,3]dioxol-4-yl)oxy]ethanol (Compound Ⅰ)
[0069]
[0070] Take a 250mL reaction bottle, add 4,6-dichloro-2-(propylthio)pyrimidin-5-amine (16.1g, 68mmol), 2-[[(3aR,4S,6R,6aS)-6-amino- 2,2-Dimethyltetrahydro-3aH-cyclopentadieno[d][1,3]-dioxol-4-yl]oxy]-1-ethanol-L-tartrate (25.0 g, 68mmol), triethylamine (68.7g, 680mmol) and ethylene glycol monomethyl ether (50mL). The resulting reaction mixture was heated to 120° C. under airtight and kept at this temperature for 40 h. It was then cooled to 30°C. The solvent was evaporated. Isopropyl acetate and water were added and the phases were separated. The aqueous phase was extracted with isopropyl acetate, and the organic phases were combined and washed with water. Dry over anhydrous magnesium sulfate. filter. The solvent was evaporated to obtain a reddish-brown oil. After a...
Embodiment 3
[0071] Example 3, 2-[((3aR,4S,6R,6aS)-6-[[5-amino-6-chloro-2-(propylthio)pyrimidin-4-yl]amino]-2,2- Preparation of Dimethyltetrahydro-3aH-Cyclopenta[d][1,3]dioxol-4-yl)oxy]ethanol (Compound Ⅰ)
[0072]
[0073] Take a 250mL reaction bottle, add 4,6-dichloro-2-(propylthio)pyrimidin-5-amine (16.1g, 68mmol), 2-[[(3aR,4S,6R,6aS)-6-amino- 2,2-Dimethyltetrahydro-3aH-cyclopentadieno[d][1,3]-dioxol-4-yl]oxy]-1-ethanol-oxalate (20.9g , 68mmol), triethylamine (103.0g, 1020mmol) and ethylene glycol monomethyl ether (161mL). The resulting reaction mixture was heated to 130° C. under airtight and kept at this temperature for 45 h. It was then cooled to 30°C. Isopropyl acetate and water were added and the phases were separated. The aqueous phase was extracted with isopropyl acetate, and the organic phases were combined and washed with water. Dry over anhydrous magnesium sulfate. filter. The solvent was evaporated to obtain a reddish-brown oil. After adding n-heptane for beating, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com