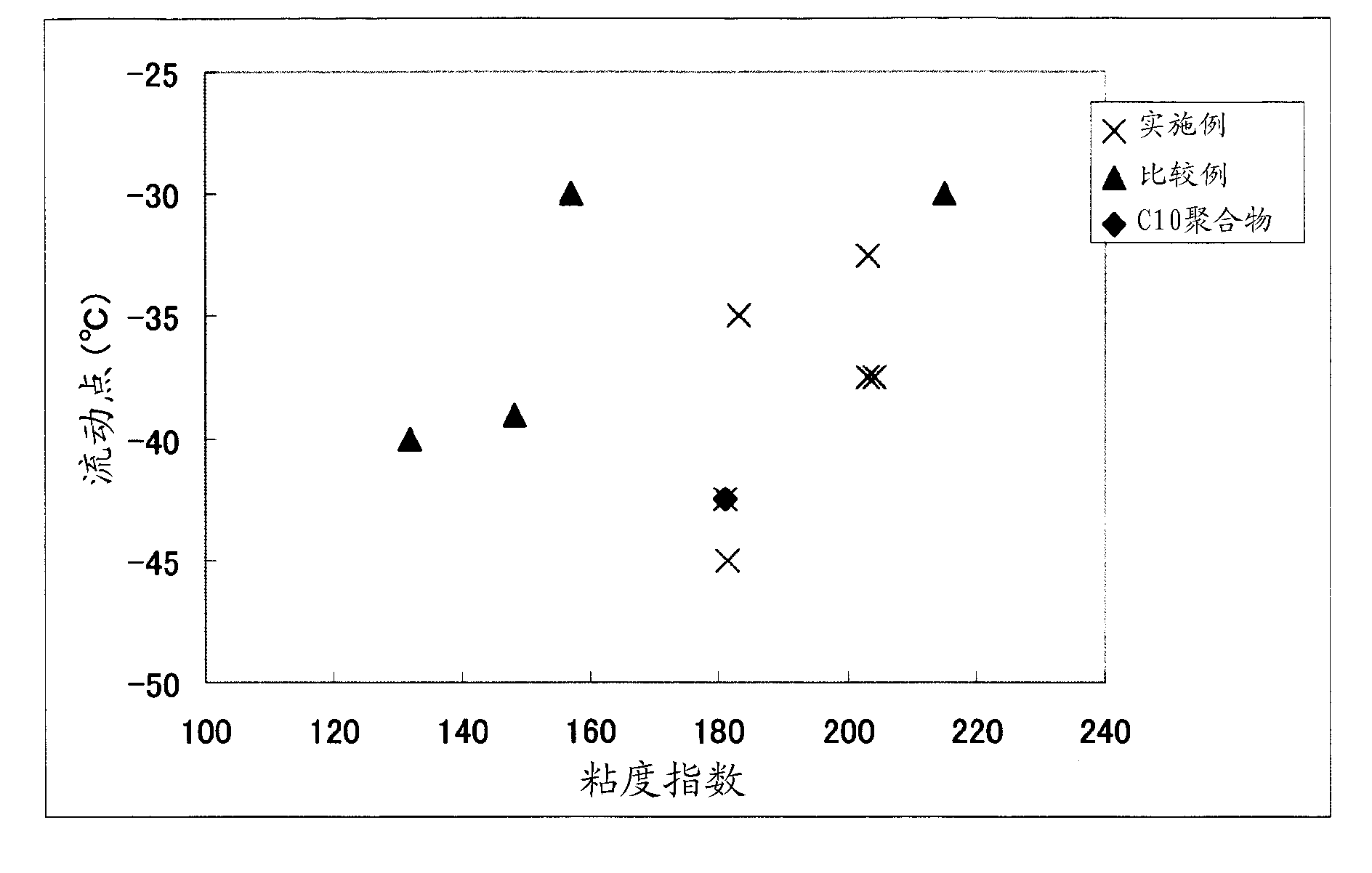
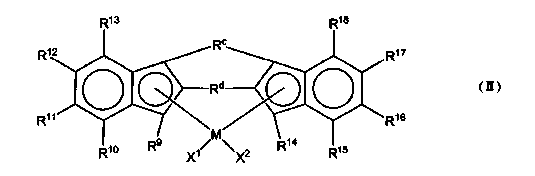
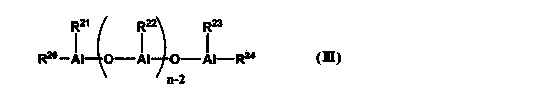1-decene/1-dodecene copolymer and lubricating-oil composition containing same
A kind of technology of dodecene and copolymer, applied in the field of 1-decene-1-dodecene copolymer and lubricating oil composition containing it, can solve the relationship between polymer structure and oxidative stability Not specifically known and other problems, to achieve the effect of excellent oxidation stability and excellent physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0110] Next, the present invention will be described in more detail using examples, but the present invention is not limited to these examples.
[0111] The evaluation of the physical properties of the α-olefin polymer was carried out by the following methods.
[0112] (1) Flow point
[0113] Measured in accordance with JIS K 2269.
[0114] (2) Kinematic viscosity and viscosity index
[0115] The kinematic viscosity is measured according to JIS K 2283. Viscosity index is calculated from kinematic viscosity according to JIS K 2283.
[0116] (3) Number average molecular weight and molecular weight distribution (Mw / Mn)
[0117] Using JASCO GPC-900 (column; TOSOH TSK-GEL MULTIPORE HXL-M (2 pieces) + Shodex KF801 (1 piece)) device, under the conditions of solvent; tetrahydrofuran, temperature; 40°C, and polystyrene conversion Get it.
[0118] (4) Meso triad fraction (mm)
[0119] Using the method described in [Macromolecules24, 2334 (1991); Polymer, 30, 1350 (1989)], using ...
manufacture example 1
[0134] Production Example 1 [Production of (1,1'-dimethylsilylene)(2,2'-dimethylsilylene)-bis(cyclopentadienyl)zirconium dichloride]
[0135] About 13.8 g (600 mmol) of metallic Na and 400 ml of dry THF (tetrahydrofuran) were added to a 1000 ml three-necked flask replaced with nitrogen, and stirred at 0°C. After 5 minutes, 1 to 2 ml of cyclopentadiene was added dropwise thereto, and when generation of hydrogen was completed, 1 to 2 ml of cyclopentadiene was added again, and the process was repeated to add a total of 50 ml (600 mmol) of cyclopentadiene. The reaction solution changed from colorless and transparent to light pink. After THF was distilled off under reduced pressure, the crystals were washed twice with hexane and dried under reduced pressure to obtain cyclopentadienyl sodium as a pink powder.
[0136] 457 ml of THF was added to 43.0 g (480 mmol) of cyclopentadienyl sodium at 0° C., followed by stirring. After cooling to -78°C, 29.2ml (480mmol) of dichlorodimethyls...
Embodiment 1-1
[0143] A stainless steel autoclave with an inner volume of 1 liter was fully dried and replaced with nitrogen, 80 ml of 1-dodecene and 320 ml of 1-decene were added, followed by 0.2 mmol of triisobutylaluminum, and the temperature was raised to 105 ℃. Put into the catalyst mixed solution prepared separately (in a 10 ml glass Schlenk bottle, add 0.1 mmol of triisobutylaluminum (2 mmol / ml toluene solution; 0.05 ml) under nitrogen atmosphere, obtain in Production Example 1 (1,1'-dimethylsilylene)(2,2'-dimethylsilylene)-bis(cyclopentadienyl)zirconium dichloride 10 μmol (10 μmol / mL of toluene solution; 1 mL) and powdered N, N-dimethylanilinium tetrakis (pentafluorophenyl) borate 0.012 mmol (11.5 mg), after stirring at room temperature for about 1 minute, add 1 -decene 0.5 ml, and further stirred at room temperature for 1 hour) After 1 ml of hydrogen 0.05 MPaG was introduced to initiate polymerization. After 60 minutes at 105°C, add 1 ml of the remaining catalyst mixed solution, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com