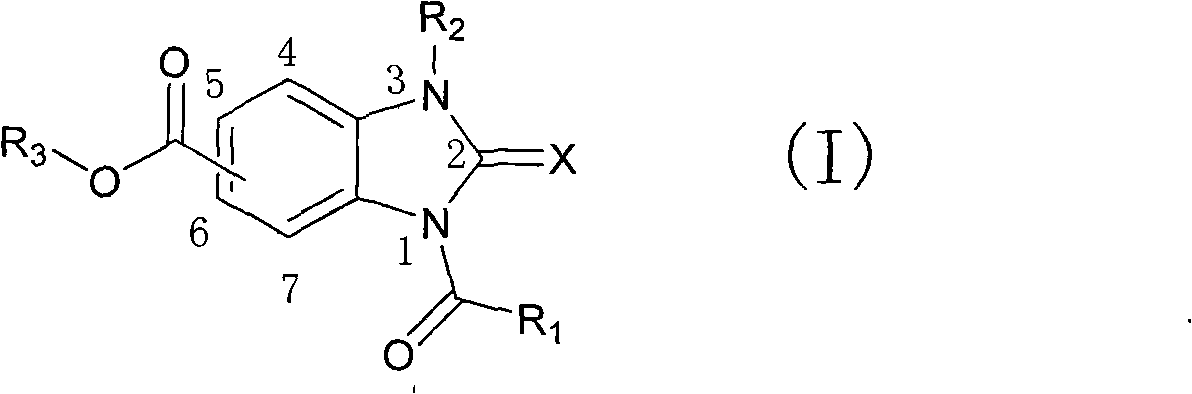
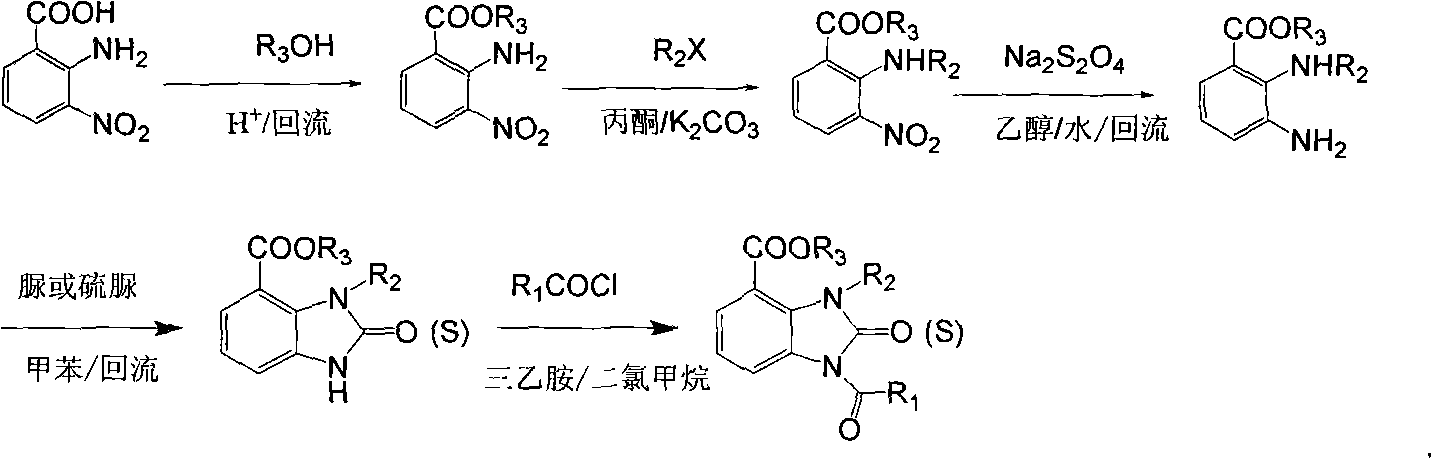
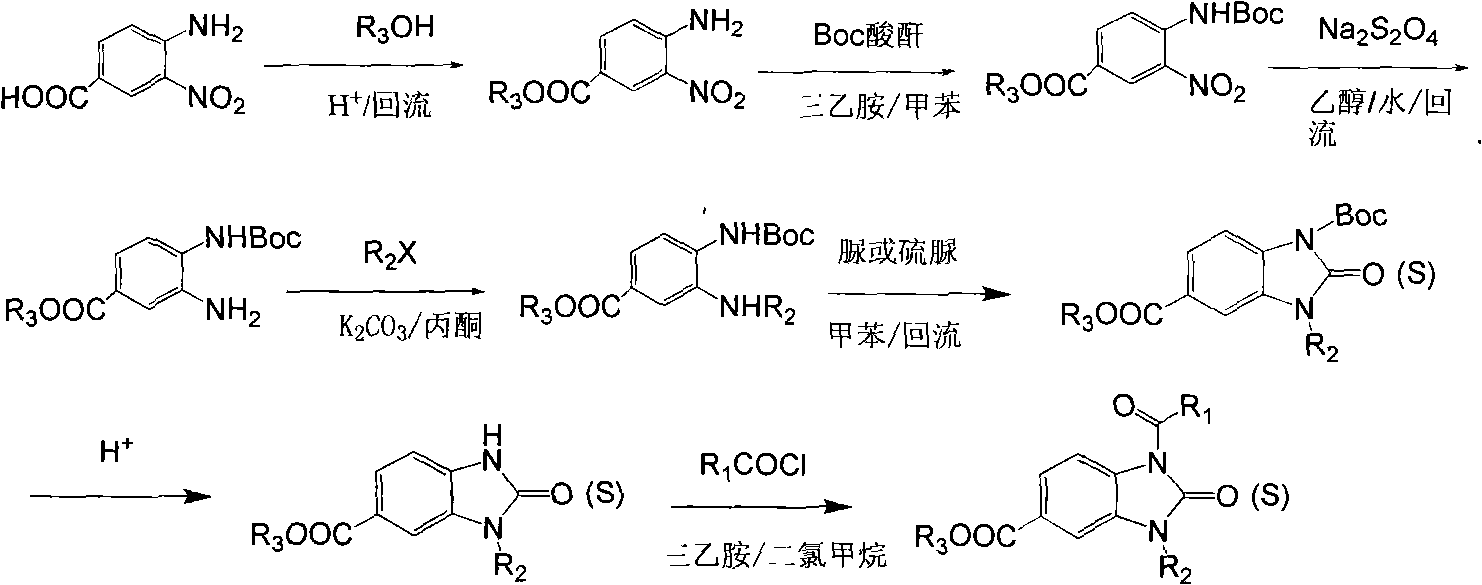
Derivative of 1-acyl benzimidazolone and use thereof as agricultural fungicide
A technology of acylbenzimidazolone and derivatives, applied in the field of new 1-acylbenzimidazolone derivatives and agricultural fungicides, can solve the problems of few research reports on benzimidazolone derivatives, and achieve strong inhibition Bacterial activity, inhibition of mycelium growth, and inhibition of spore germination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The following specific preparation examples and biological examples are helpful to understand the present invention, but do not limit the content of the present invention.
[0043] The following preparation examples 1-18 further illustrate the preparation method of the compound of general formula (I) and the preparation of the present application, but are not limited thereto.
Embodiment 1
[0045] Synthesis of 3-isopropyl-2-oxo-1-pentanoyl-2,3-dihydro-1H-benzimidazole-4-carboxylic acid ethyl ester
[0046]
[0047] Weigh Ia (1.82g, 10mmol), ethanol (4.6g, 100mmol), 30mL of toluene was placed in a 100mL eggplant-shaped flask, then 3mL of concentrated sulfuric acid was added and heated to reflux for 3h, after cooling to room temperature, saturated sodium bicarbonate was added to neutralize the reaction The liquid was transferred to a separatory funnel, extracted with ethyl acetate (20 mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain Intermediate Ib (1.81 g) with a yield of 86.0%;
[0048] Weigh Ib (1.05g, 5mmol), 1.68g (30mmol) potassium hydroxide, 10mL dimethylformamide (DMF) in a 100mL eggplant flask, stir at room temperature for 30min, add 1.02g (6mmol) isopropane iodide, Continue to stir the reaction at room temperature for 15h. Add 50 mL of ethyl acetate / water (volume ratio 2 / 1)...
Embodiment 2
[0054] Synthesis of 3-allyl-1-(3-methylbenzoyl)-2-thio-2,3-dihydro-1H-benzimidazole-5-carboxylic acid ethyl ester
[0055]
[0056] Weigh IIa (1.82g, 10mmol), ethanol (4.6g, 100mmol), 30mL of toluene is placed in a 100mL eggplant-shaped bottle, then 3mL of concentrated sulfuric acid is added and heated to reflux for 3h, and intermediate IIb is obtained according to the method in Example 1 ( 1.92g), yield 91.4%;
[0057] Weigh IIb (1.05 g, 5 mmol), Boc anhydride (1.31 g, 6 mmol), 30 mL of toluene in a 100 mL eggplant-shaped flask, add triethylamine (1 mL) dropwise at room temperature, and continue stirring at room temperature for 12 h after the addition. Add 50 mL of ethyl acetate / water (volume ratio 2 / 1), stir for 10 min, transfer to a separatory funnel and let stand for layers, extract the aqueous phase with 10 mL of ethyl acetate again, combine the organic phases, and concentrate under reduced pressure to obtain an intermediate body IIc. Transfer to a 250mL three-necked...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com