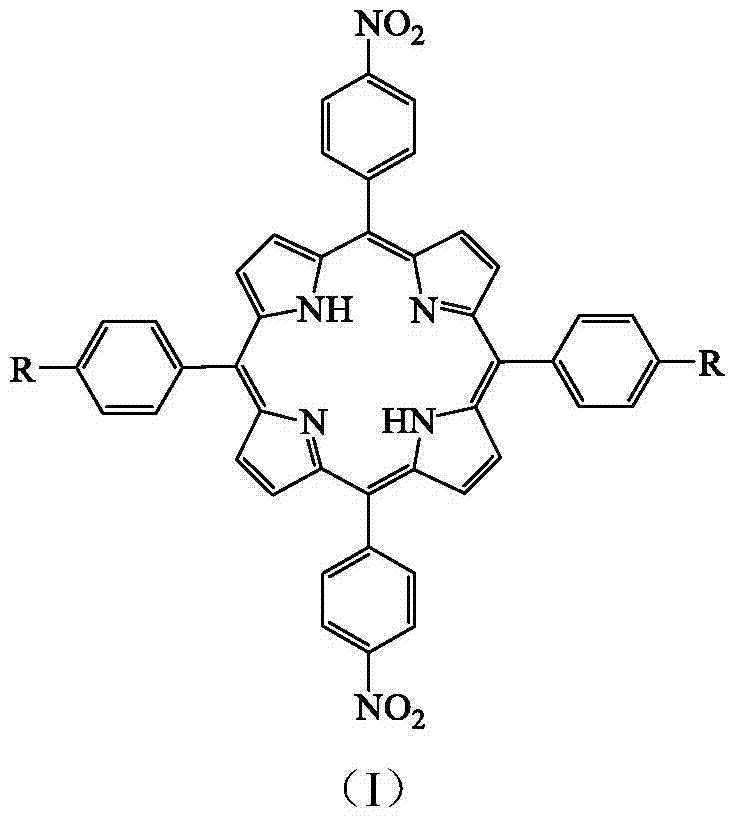
Method for preparing asymmetric 5,15-bis(p-nitrophenyl)-10, 20-diphenyl porphyrin compound
A technology of p-nitrophenyl and diphenyl, which is applied in the field of preparation of asymmetric porphyrin compounds, can solve problems such as unfavorable regulation of acidity, polarity and viscosity of reaction medium, complicated preparation process operation, high cost of solvent recovery, etc. , to achieve the effects of easy regulation of the reaction process, full utilization of solvent resources, and easy control of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
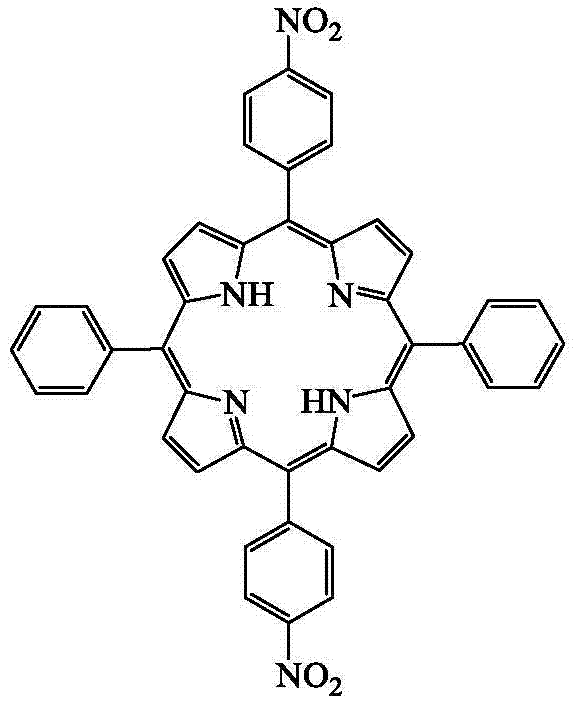
[0035] Example 1: Preparation of 5,15-di-p-nitrophenyl-10,20-diphenylporphyrin compound
[0036] In a 25mL three-necked flask equipped with a magnetic rotor, add 5mL nitrobenzene and 2.5mL acetic acid in sequence, preheat at 100°C, then add 1mmol benzaldehyde and 1mmol5-(4-nitrophenyl)dipyrromethane, Stirring at 100°C for 60 min, and then rotary evaporation under reduced pressure to remove nitrobenzene and acetic acid, the reaction mixture was separated by column chromatography to obtain 5,15-di-p-nitrophenyl-10,20-diphenylporphyrin compound. The yield thereof was 32%. Its structural formula is as follows.
[0037]
[0038] UV-vis (CH 2 Cl 2 )λ max / nm(logε):420.1(5.48),515.9(4.41),551.9(4.20),590.4(4.07),646.5(4.03).IR(KBr):3317.95,3101.63,3070.84,1595.00,1558.79,1516.04,1472.5 1400.30, 1344.14, 1223.13, 1186.39, 1107.42, 1018.78, 965.16, 847.26, 801.04, 746.92, 725.72, 700.42cm -1 .HRMS(ESI):m / z[M+H] + calcd for C 44 h 28 N 6 o 4+H:705.2245,found:705.2183. 1 H...
Embodiment 2
[0039] Example 2: Preparation of 5,15-di-p-nitrophenyl-10,20-diphenylporphyrin compound
[0040] In a 25mL three-necked flask equipped with a magnetic rotor, add 10mL of nitrobenzene and 5mL of acetic acid in sequence, preheat at 70°C, then add 1mmol of benzaldehyde and 1mmol of 5-(4-nitrophenyl)dipyrromethane, Stir at ℃ for 60 min, then nitrobenzene and acetic acid are removed by rotary evaporation under reduced pressure, and the reaction mixture is separated by column chromatography to obtain 5,15-di-p-nitrophenyl-10,20-diphenylporphyrin compound. The yield thereof was 11%. See Example 1 for its structural formula and characterization data.
Embodiment 3
[0041] Example 3: Preparation of 5,15-di-p-nitrophenyl-10,20-diphenylporphyrin compound
[0042] In a 25mL three-necked flask equipped with a magnetic rotor, add 10mL of nitrobenzene and 5mL of acetic acid in sequence, preheat at 100°C, then add 1mmol of benzaldehyde and 1mmol of 5-(4-nitrophenyl)dipyrromethane, and Stir at ℃ for 60 min, then nitrobenzene and acetic acid are removed by rotary evaporation under reduced pressure, and the reaction mixture is separated by column chromatography to obtain 5,15-di-p-nitrophenyl-10,20-diphenylporphyrin compound. Its yield is 30%. See Example 1 for its structural formula and characterization data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com