Method for high-efficiency purification of quantum dot-IgG monoclonal antibody conjugate
A monoclonal antibody and quantum dot technology, applied in the biological field, can solve problems such as harsh operating conditions, complicated process, and low yield, and achieve the effect of low equipment requirements, simple operation, and high-efficiency separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 water-soluble quantum dot anti-aflatoxin B 1 Monoclonal antibody conjugates and purification process
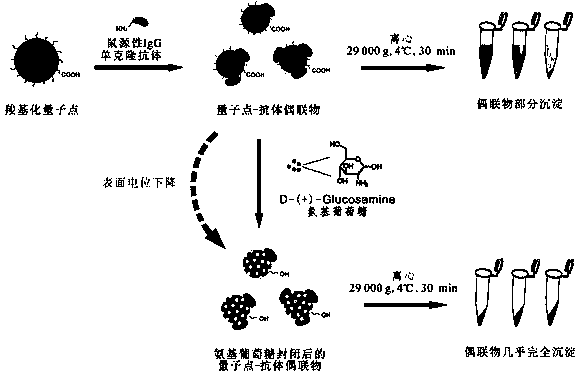
[0032] Take 5 mL of commercial carboxylated water-soluble quantum dots (concentration: 50 nmol / L) and mix with an equal volume of 0.05 mol / L borate buffer at pH 6.0; respectively add 1- Ethyl-(3-dimethylaminopropyl) carbodiimide and N-hydroxysulfosuccinimide were reacted at 37°C for 2 hours; anti-aflatoxin was added with a molar ratio of 10:1 to quantum dots B 1 Monoclonal antibody solution, after adjusting the pH of the solution to 7.5 with 1 M NaOH solution, react at room temperature for 3 hours; finally add 2% glucosamine to the solution, and further adjust the pH to 4.5 with 1 M HCl solution. 18,000 rpm (about 29,000 g) centrifuge at 4°C for 30 min, discard the supernatant, and precipitate with 25% glycerol, 0.01% NaN 3 0.05 mol / L phosphate buffer (pH 7.0-7.5) was dissolved to obtain free anti-aflatoxin B 1 Monoclonal antibody against water-soluble...
Embodiment 2
[0033] Example 2 Water-soluble quantum dot anti-ochratoxin monoclonal antibody conjugate and purification process
[0034] Take 5 mL of commercial carboxylated water-soluble quantum dots (concentration: 50 nmol / L) and mix with an equal volume of 0.05 mol / L borate buffer at pH 6.0; respectively add 1 -Ethyl-(3-dimethylaminopropyl) carbodiimide and N-hydroxysulfosuccinimide, reacted at 37°C for 2 hours; Toxin monoclonal antibody solution, after adjusting the pH of the solution to 8.0 with 1 M NaOH solution, react at room temperature for 3 hours; finally add 2.5% glucosamine to the solution, and further adjust the pH to 4.5 with 1 M HCl solution. 18,000 rpm (about 29,000 g) at 4°C for 30 min, discard the supernatant, and use 25% glycerol, 0.01% NaN for precipitation 3 The water-soluble quantum dot anti-ochratoxin monoclonal antibody conjugate without free anti-ochratoxin monoclonal antibody was obtained by dissolving in 0.05 mol / L phosphate buffer (pH 7.0-7.5). After the water-...
Embodiment 3
[0035] Example 3 Water-soluble carboxyl quantum dot anti-zearalenone monoclonal antibody conjugate and purification process
[0036] Take 5 mL of commercial carboxylated water-soluble quantum dots (concentration: 50 nmol / L) and mix with an equal volume of 0.05 mol / L borate buffer at pH 5.5; respectively add 1- Ethyl-(3-dimethylaminopropyl) carbodiimide and N-hydroxysulfosuccinimide were reacted at 37°C for 2 hours; the molar ratio of quantum dots was 3:1 by adding anti-zeb For enone monoclonal antibody solution, adjust the pH of the solution to 8.0-9.0 with 1 M NaOH solution, react at room temperature for 3 hours; finally add 2.5% glucosamine to the solution, and further adjust the pH to 4.5 with 1 M HCl solution. Centrifuge at 18,000 rpm (about 29,000 g) at 4°C for 30 min, discard the supernatant, and use 25% glycerol, 0.01% NaN for precipitation 3 The water-soluble quantum dot anti-zearalenone monoclonal antibody conjugate without free anti-zearalenone monoclonal antibody w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com