Preparation method of nitrogen-doped graphene material
A nitrogen-doped graphene and graphite technology, applied in graphene, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of harsh operating conditions, high alkalinity, and single nitrogen species in nitrogen-doped graphene , to achieve the effect of low cost and high nitrogen content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
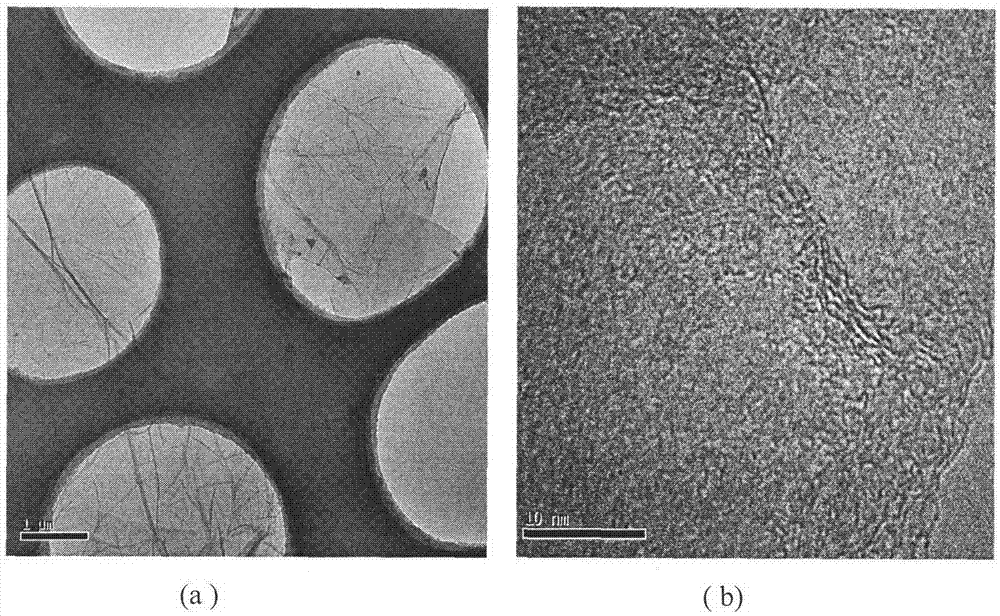
Image
Examples
Embodiment 1
[0027] (1) Preparation of graphite oxide: Add 240mL of concentrated sulfuric acid and 30mL of concentrated phosphoric acid into a three-neck flask, add 6g of graphite powder, then slowly add 18g of potassium permanganate, stir at 50°C for 6-24h, then add 400mL of water . At this point the temperature rose sharply to 98°C and was maintained for 30 minutes. Add 3 mL of 30% hydrogen peroxide.
[0028] (2) Purification of graphite oxide: the turbid liquid obtained in (1) was left to stand, and then decanted. Decant four times with deionized water, then six times with ethanol. Graphite oxide can be obtained by vacuum drying at 80°C for 24 hours.
[0029] (3) Preparation of graphene oxide: 10 g of graphite oxide was weighed and added to 1 L of deionized water and stirred slowly. After stirring for 30 minutes, ultrasonically disperse for 30 minutes. The obtained aqueous solution is the aqueous solution of graphene oxide. The pH value of the aqueous solution is between 4 and 5. ...
Embodiment 2
[0036] The difference between this example and example 1 is that the amine used is 70% ethylamine, the amount added is 26g, and the obtained sample is recorded as EAGO.
[0037] All the other contents are the same as those described in Example 1.
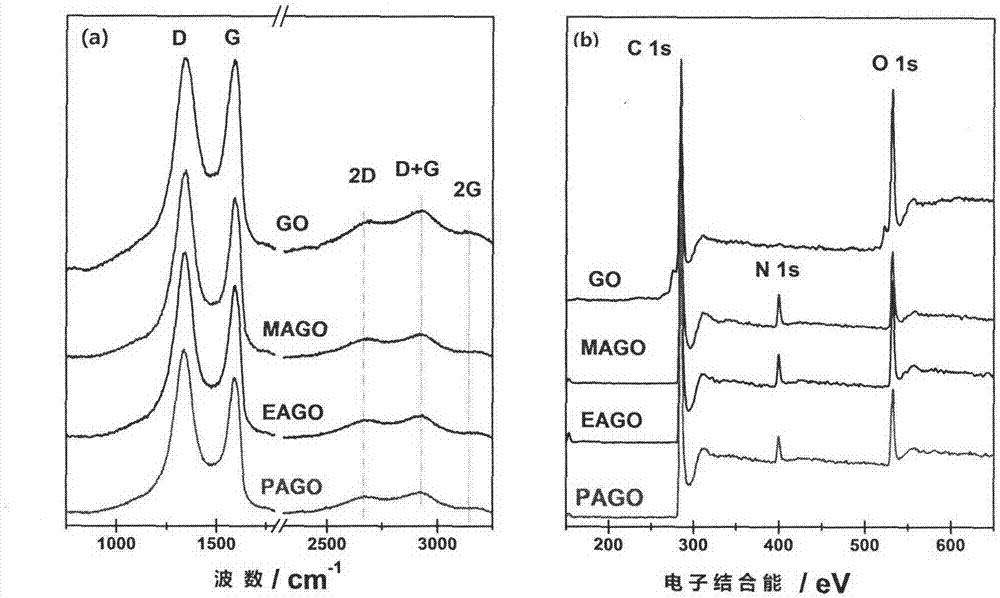
[0038] The Raman spectrum and XPS spectrogram of gained nitrogen-doped graphene are as figure 2 shown.
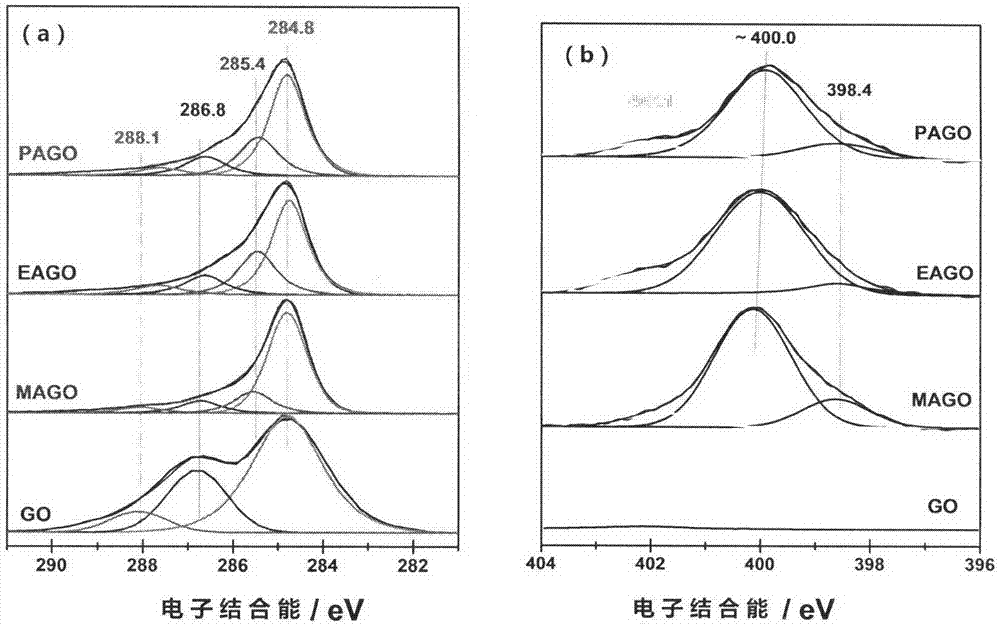
[0039] The high-resolution C 1s XPS spectrum of gained nitrogen-doped graphene and the high-resolution N 1s XPS spectrum are as follows image 3 shown.
Embodiment 3
[0041] The difference between this example and Example 1 is that the amine used is 30% methylamine, the amount added is 40 g, and the obtained sample is marked as MAGO.
[0042] All the other contents are the same as those described in Example 1.
[0043] The Raman spectrum and XPS spectrogram of gained nitrogen-doped graphene are as figure 2 shown.
[0044]The high-resolution C 1s XPS spectrum of gained nitrogen-doped graphene and the high-resolution N 1s XPS spectrum are as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com