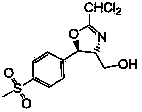
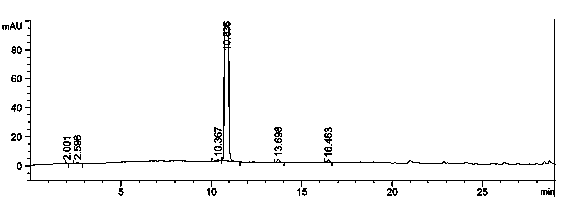
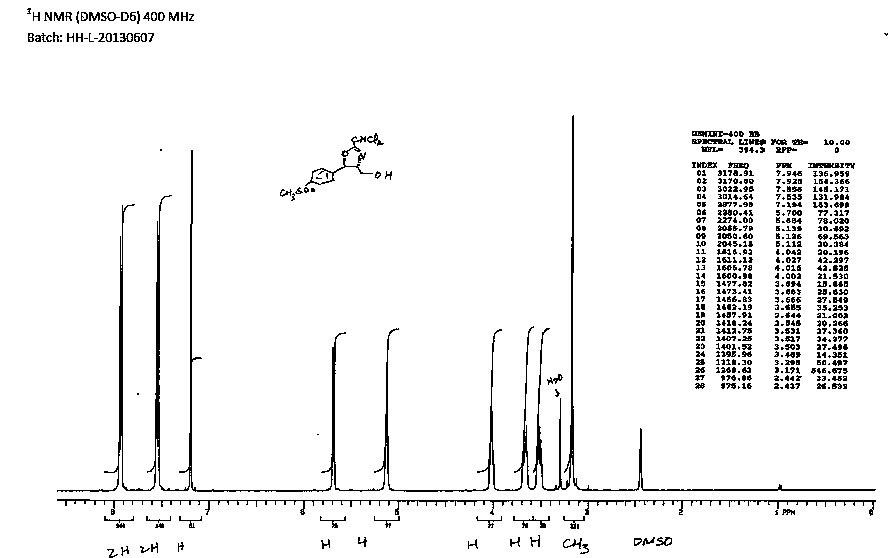
Preparation method of florfenicol oxazoline intermediate
A technology of florfenicol oxazoline and intermediates, which is applied in the field of drug synthesis, can solve the problems of prone to punching and explosion, unsafety, and the inability of one-time input of reducing agents, etc., and achieves easy operation, slow speed, and difficult The effect of punching and explosion accidents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In parts by weight, weigh 550 parts of methanol, add it into the reaction bottle, weigh 0.5 part of sodium methoxide, add it into methanol and stir to dissolve. Weigh 95 parts of D-p-thymphenylphenylserine ethyl ester, stir and add it into the reaction flask at room temperature. Weigh 20 parts of sodium borohydride, add it into the reaction bottle at room temperature at one time, and then react at a temperature of 50° C. for 6 hours. After the reaction, methanol was concentrated under reduced pressure, then 150 parts of glycerin was added, and the pH was adjusted to 6-7 with glacial acetic acid; 42 parts of dichloroacetonitrile was weighed and added dropwise to the reaction solution, and the temperature was raised to 50°C for 18 hours. After the reaction, 250 parts of 25% ethanol aqueous solution was added dropwise to the reaction solution with stirring and dropping to room temperature, followed by suction filtration, washing and drying to obtain 103 parts of white powd...
Embodiment 2
[0030] In parts by weight, weigh 600 parts of ethanol, add it into the reaction bottle, weigh 0.7 part of sodium ethylate, add it into the ethanol and stir to dissolve. Weigh 100 parts of D-p-thymphenylphenylserine ethyl ester, stir and add it into the reaction flask at room temperature. Weigh 18 parts of sodium borohydride, add it into the reaction bottle at room temperature at one time, and then react at a temperature of 50° C. for 6 hours. After the reaction, ethanol was concentrated under reduced pressure, 170 parts of glycerin was added, and the pH was adjusted to 6-7 with glacial acetic acid. Weighed 45 parts of dichloroacetonitrile and added dropwise to the reaction solution, and heated to 50°C for 18 hours. , after the reaction, stir and drop 250 parts of 25% ethanol aqueous solution in the reaction solution, suction filter after cooling down to room temperature, wash and dry to obtain 102 parts of white powdery florfenicol oxazoline intermediate, molar yield 86.7 %,...
Embodiment 3
[0032] In parts by weight, weigh 600 parts of methanol, add it into the reaction bottle, weigh 0.7 parts of potassium tert-butoxide, add it into methanol and stir to dissolve. Weigh 100 parts of D-p-thymphenylphenylserine ethyl ester, stir and add it into the reaction flask at room temperature. Weigh 18 parts of sodium borohydride, add it into the reaction bottle at room temperature at one time, and then react at a temperature of 48° C. for 6 hours. After the reaction, the methanol was concentrated under reduced pressure, 180 parts of glycerin was added, and the pH was adjusted to 6-7 with glacial acetic acid. Weighed 40 parts of dichloroacetonitrile and added dropwise to the reaction solution, and heated to 50°C for 18 hours. After the reaction, 250 parts of 25% ethanol aqueous solution was added dropwise to the reaction solution with stirring and dropping to room temperature, followed by suction filtration, washing, and drying to obtain 102 parts of white powdery florfenico...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com