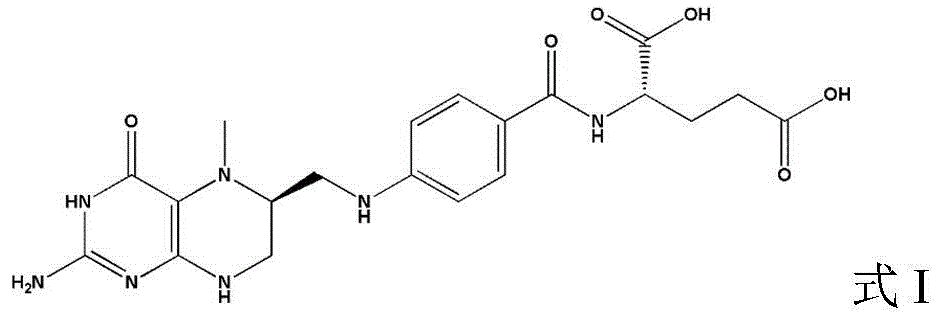
A 5-methyl-(6s)-tetrahydrofolate crystal form a and its preparation method
A technology of tetrahydrofolate and methyl group, applied in the direction of organic chemistry, etc., can solve the problems such as complicated operation of the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
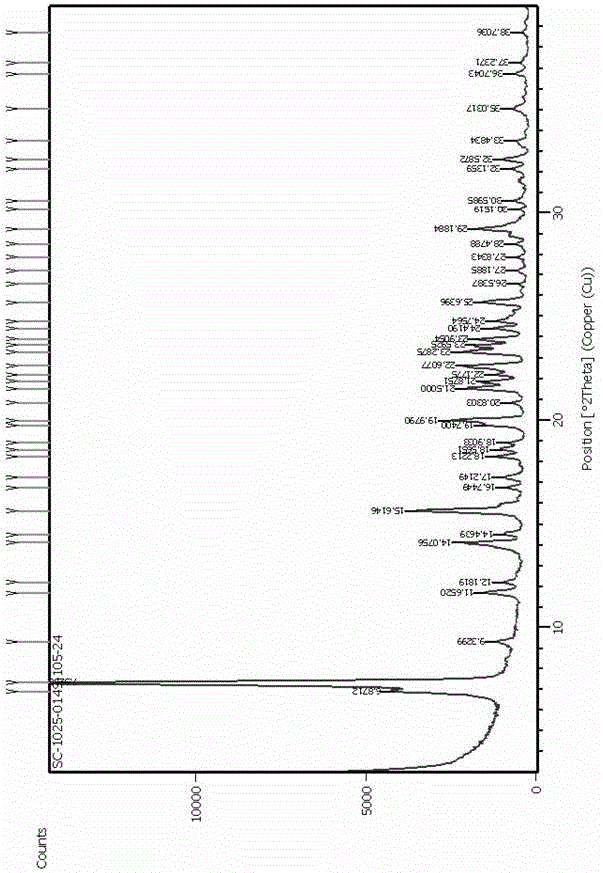
[0026] Example 1 [Stability]
[0027] Liquid phase conditions:
[0028] Column: Hypersil-ODS, 5μm; 250 x 4.6 mm (Thermo Hypersil Keystone equivalent)
[0029] Mobile Phase A: Dissolve 7.80g of 0.05 mol NaH 2 PO 4 2H 2 O was dissolved in 1000 ml water, adjusted to pH 6.5 with 32% NaOH, filtered and degassed
[0030] Mobile phase B: dissolve 5.07 g 0.03 mol NaH 2 PO 4 2H 2 O was dissolved in 350 ml methanol, adjusted to pH 8.0 with 32% NaOH, filtered, degassed
[0031] Time 0-14 min mobile phase A: mobile phase B (volume ratio) = 100-45: 0-55; 14-17 min mobile phase A: mobile phase B (volume ratio) = 45- 0: 55-100; 17 -24 min mobile phase A: mobile phase B (volume ratio) = 0: 100; 24.01-33 min mobile phase A: mobile phase B (volume ratio) = 100: 0
[0032] Injection volume: 10 μl
[0033] Flow rate: 1.1ml / min
[0034] Column temperature: 32°C
[0035] Detection wavelength: 280 nm.
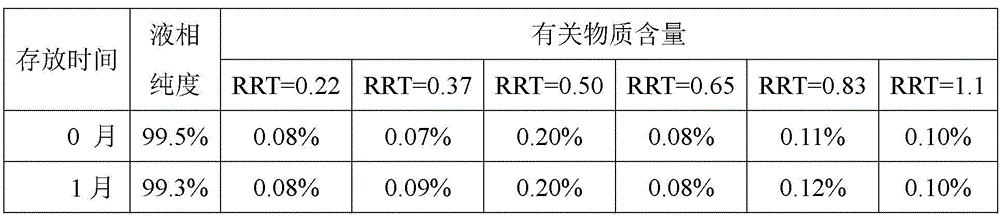
[0036] The liquid phase purity and related substances of 5-methyl-(6S)-tetrahydrofol...
Embodiment 2
[0039] Example 2 [Solubility]
[0040] The solubility of 5-methyl-(6S)-tetrahydrofolate crystal Form A in water at 25°C was compared with the crystal form obtained by the prior art (WO2008144953). See the table below for specific data.
[0041] Sample source
Embodiment 3
[0042] Example 3 5-methyl-(6S)-tetrahydrofolate crystal Form A
[0043] 2.0 g of 5-methyl-(6S)-tetrahydrofolic acid with a liquid phase purity of 96.3% was added to 50 ml of benzenesulfonic acid aqueous solution at 60°C (5.0 g of benzenesulfonic acid, 50 ml of water). The liquid is dissolved and clarified. The temperature was lowered to 20°C for crystallization for 2 hours, and the solid was vacuum-dried at 40°C to obtain 1.90 g of white 5-methyl-(6S)-tetrahydrofolate crystal Form A. The purity is 99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com