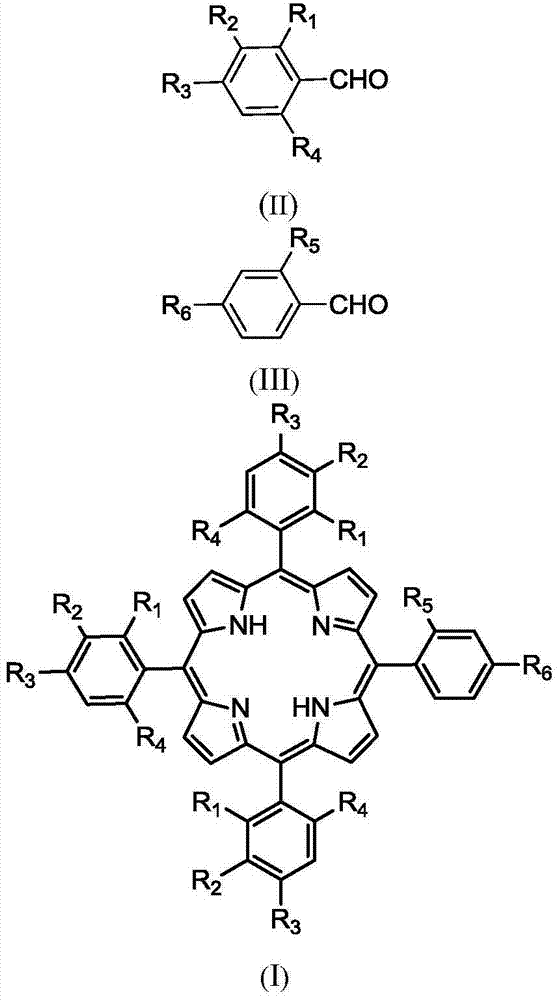
Preparation method of A3B type asymmetric porphyrin compounds
An asymmetric and compound technology, which is applied in the field of preparation of A3B asymmetric porphyrin compounds, can solve the problems that the reaction concentration is not allowed to be too high, the synthesis and separation operations are cumbersome, and the reaction time is long, so as to save separation and operation costs and energy consumption, saving energy and operating costs, the effect of short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
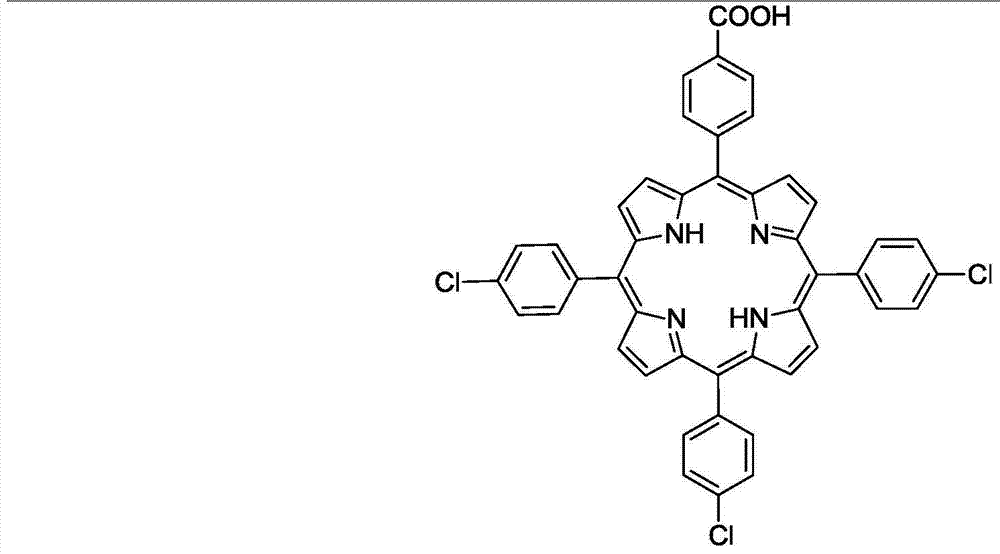
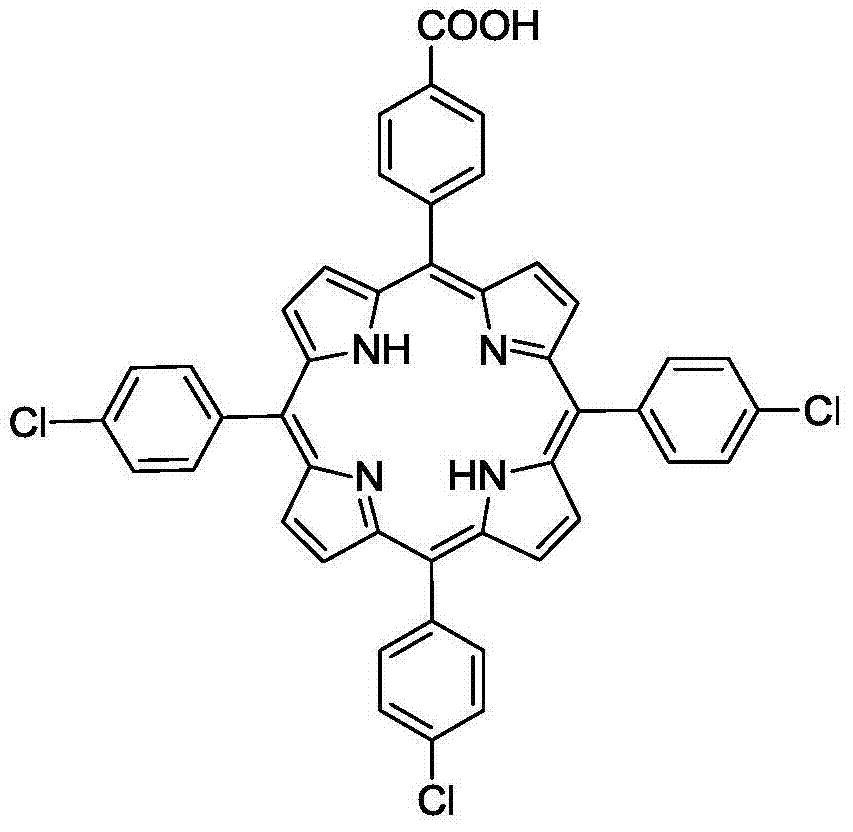
[0035] In a three-necked flask equipped with a reflux condensing device, mix 20mmol of 4-chlorobenzaldehyde and 40mmol of pyrrole and add them to a solution containing 75ml of acetic acid, 50ml of xylene, and 10ml of acetic anhydride, then add 150uL of trifluoroacetic acid, and at 125°C After reacting for 1min, add 10mmol 4-chlorobenzaldehyde and 10mmol 4-carboxybenzaldehyde at the same time, stop the reaction after continuing the reaction for 1.5h, let stand to cool to room temperature, add 20ml of methanol, let stand overnight, then filter with Buchner funnel to obtain the crude product, The crude product was separated by column chromatography to obtain 5-(4-carboxyphenyl)-10,15,20(4-chlorophenyl)porphyrin compound with a yield of 17%. Its structure and characterization data are as follows:
[0036]
[0037] UV-vis(CH2Cl2)λmax / nm: 422.4,515.1,550.1,589.8,645.9.IR(KBr):3314,1694,1557-1472,1016,996,798cm-1.HRMS(ESI):m / z[M +H]+calcd for C44H28N6O4+H:705.2245,found:705.2183....
Embodiment 2
[0039]In a three-necked flask equipped with a reflux condensing device, mix 20mmol of 4-chlorobenzaldehyde and 40mmol of pyrrole and add to a solution containing 75ml of propionic acid, 50ml of xylene, and 10ml of acetic anhydride, then add 150uL of trifluoroacetic acid, After reacting for 1min, add 10mmol 4-chlorobenzaldehyde and 10mmol 4-carboxybenzaldehyde at the same time, stop the reaction after continuing the reaction for 1.5h, let it cool down to room temperature, add 20ml of methanol, let it stand overnight, and then filter it with a Buchner funnel to obtain the crude product , the crude product was separated by column chromatography to obtain 5-(4-carboxyphenyl)-10,15,20(4-chlorophenyl)porphyrin compound with a yield of 15%. Its structure and characterization data are as follows:
[0040]
[0041] UV-vis(CH2Cl2)λmax / nm: 422.4,515.1,550.1,589.8,645.9.IR(KBr):3314,1694,1557-1472,1016,996,798cm-1.HRMS(ESI):m / z[M +H]+calcd for C44H28N6O4+H:705.2245,found:705.2183.1H N...
Embodiment 3
[0043] In a three-necked flask equipped with a reflux condensing device, mix 20mmol 4-chlorobenzaldehyde and 40mmol pyrrole and add it to a solution containing 75ml butyric acid, 50ml xylene, and 10ml acetic anhydride, then add 150uL trifluoroacetic acid, and heat the mixture at 125°C After reacting for 1min, add 10mmol 4-chlorobenzaldehyde and 10mmol 4-carboxybenzaldehyde at the same time, stop the reaction after continuing the reaction for 1.5h, let it cool down to room temperature, add 20ml of methanol, let it stand overnight, and then filter it with a Buchner funnel to obtain the crude product , the crude product was separated by column chromatography to obtain 5-(4-carboxyphenyl)-10,15,20(4-chlorophenyl)porphyrin compound with a yield of 13%. Its structure and characterization data are as follows:
[0044]
[0045] UV-vis(CH2Cl2)λmax / nm: 422.4,515.1,550.1,589.8,645.9.IR(KBr):3314,1694,1557-1472,1016,996,798cm-1.HRMS(ESI):m / z[M +H]+calcd for C44H28N6O4+H:705.2245,found...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com