Organic electrophosphorescent material and its preparation method and application
A compound and fluorine atom technology, applied in the field of organic electrophosphorescent materials and their preparation, can solve the problems of small luminescence contribution and difficulty in improving luminous efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
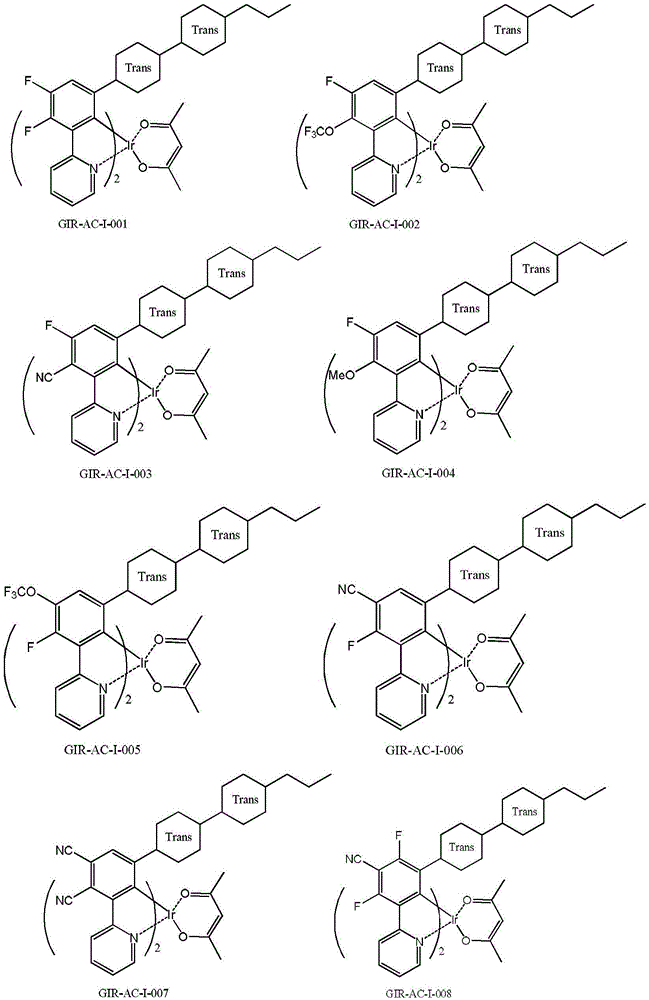
[0138] Example 1. Preparation of Compound GIR-AC-I-001 (Method 1)
[0139]
[0140] 1.0g (0.5mmol) of compound G-2, 98mg (1mmol) of acetylacetone and 519mg (5mmol) of anhydrous sodium carbonate were dispersed in 40ml of acetonitrile and 40ml of chloroform, under the protection of nitrogen, the temperature was refluxed to carry out the substitution reaction After 24 hours, cool to room temperature, pour the reaction solution into water, extract with DCM, dry the organic phase, filter, concentrate the filtrate to dryness under reduced pressure, and separate and purify the residue through a silica gel column to obtain 650 mg of compound GIR-AC-I-001. yellow solid.
[0141] Experimental data:
[0142] (1) 1 HNMR (δ, CDCl 3 ): 0.08~0.10 (1H, m); 0.12~0.18 (3H, m); 0.53~0.58 (1H, d); 0.73~0.90 (10H, m); 0.96~1.42 (15H, m); 1.64~1.78 (5H, m); 6.58-6.65 (1H, q); 6.96-7.01 (1H, t); 7.75-7.79 (1H, t); 8.21-8.33 (2H, m). It is confirmed that the substance obtained by the reaction...
Embodiment 2
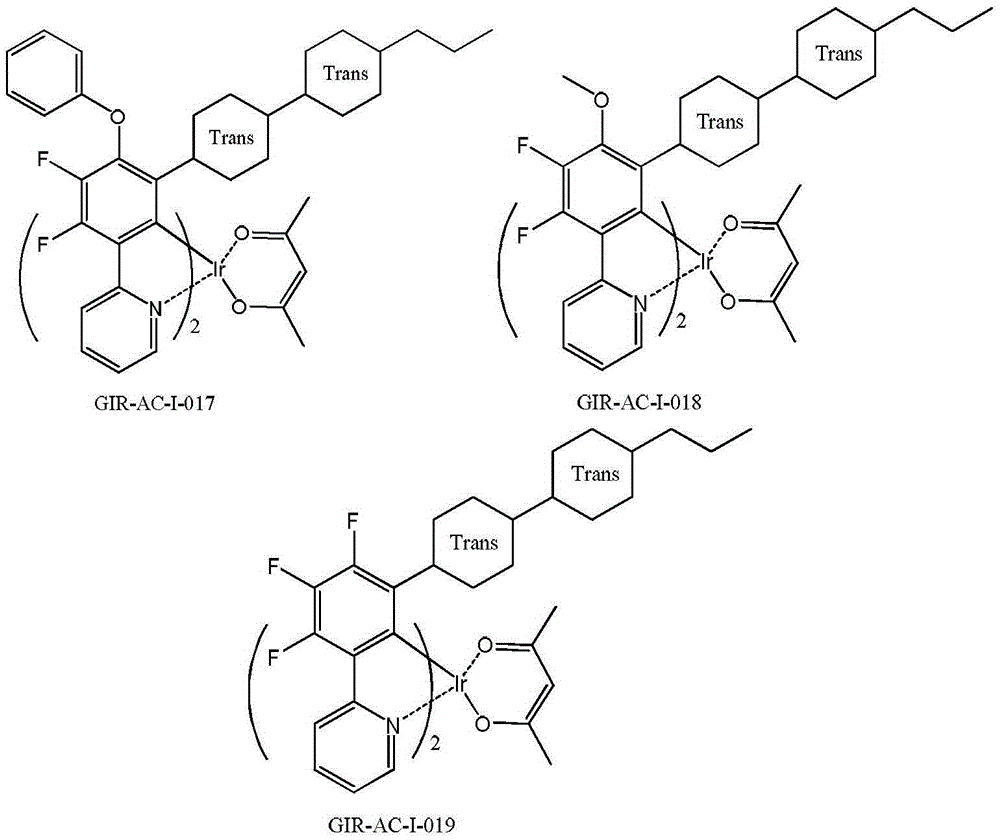
[0146] Example 2, Preparation of Compound GIR-AC-II-001 (Method 3)
[0147]
[0148] Stir and disperse 1.08g of the compound shown in G-2 and 3.97g of the compound shown in G-1 with 50ml of glycerin. Under the protection of nitrogen, raise the temperature to 180°C, stir for 8 hours for substitution reaction, cool to room temperature, pour the reaction solution into 200ml 1N dilute hydrochloric acid, filter with suction, wash the filter cake with water, separate and purify the obtained solid with a silica gel column to obtain 0.66 g of GIR-AC-I-001 as a white solid.
[0149] Experimental data:
[0150] (1) 1 HNMR (δ, CDCl 3 ): 0.11~0.18 (3H, m); 0.85~1.92 (20H, m); 2.43 (2H, m); 3.69 (2H, m); 6.58~6.65 (1H, q); ); 7.75~7.79 (1H,t); 8.21~8.33 (2H,m). It is confirmed that the substance obtained by the reaction is indeed the compound GIR-AC-II-001;
[0151] (2) Glass transition temperature (DSC): /
[0152] (3) UV maximum absorption wavelength (DCM): 325nm, 378nm;
[015...
Embodiment 3
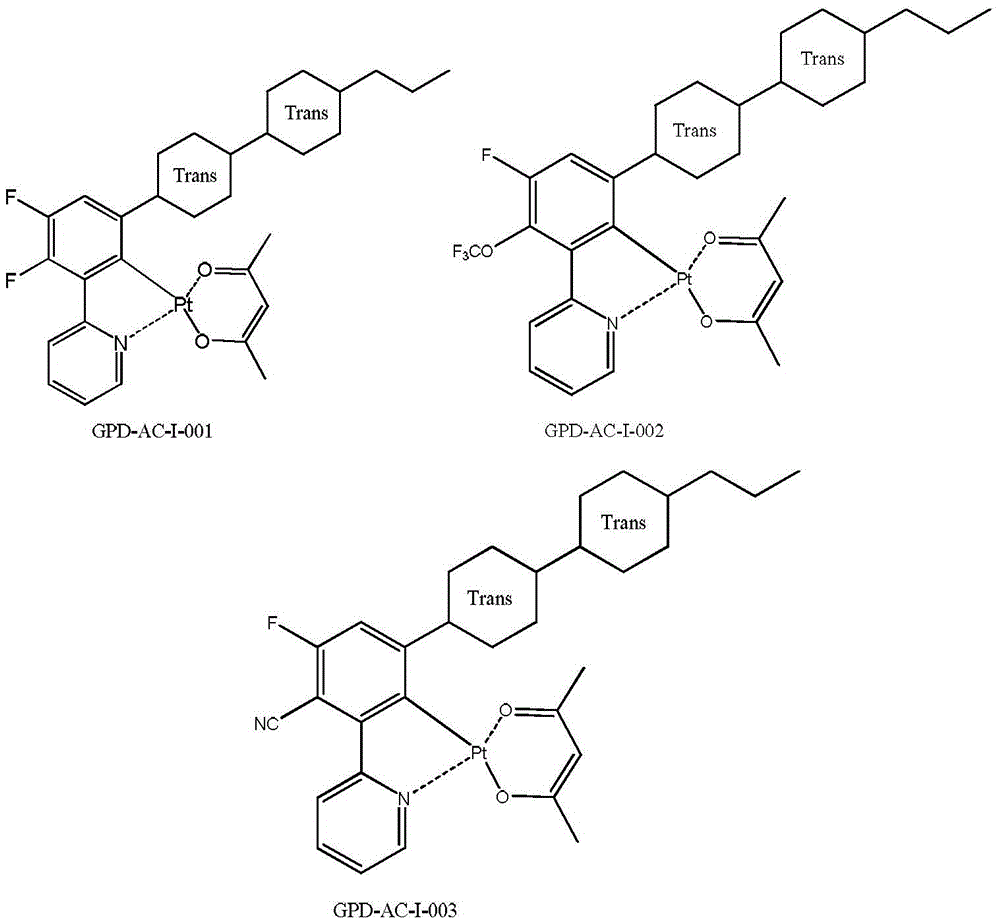
[0154] Preparation of Example 3 Compound GIR-AP-I-001 (Method 2)
[0155]
[0156] 2.04g of compound G-2 and 707mg of 2-pyridinecarboxylic acid, 324mg of anhydrous potassium carbonate and 50ml of 1,4-dioxane were heated and refluxed to carry out the substitution reaction for 8 hours, concentrated to dryness under reduced pressure, and the residue was washed with silica gel Column separation and purification yielded 1.1 g of compound GIR-AP-I-001 as a yellow solid.
[0157] Experimental data:
[0158] (1) 1 HNMR (δ, CDCl 3 ): 0.12~0.18 (6H, m); 0.84~1.94 (40H, m); 2.45 (4H, m); 3.71 (4H, m); 6.58~6.68 (2H, q); ); 7.65~7.79 (6H,t); 8.21~8.49 (4H,m). It is confirmed that the substance obtained by the reaction is indeed the compound GIR-AP-I-001;
[0159] (2) glass transition temperature (DSC): / ;
[0160] (3) UV maximum absorption wavelength (DCM): 248nm, 311nm, 378nm;
[0161] (4) Phosphorescence emission wavelength (DCM): 445nm, 510nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com