Method for processing waste sulfuric acid in graphene production process
A technology of production process and treatment method, applied in the field of comprehensive utilization of industrial waste sulfuric acid, can solve problems such as environmental pollution, consumption of large manpower and material resources, waste of resources, etc., and achieve the effects of improving environmental quality, eliminating pollution sources and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
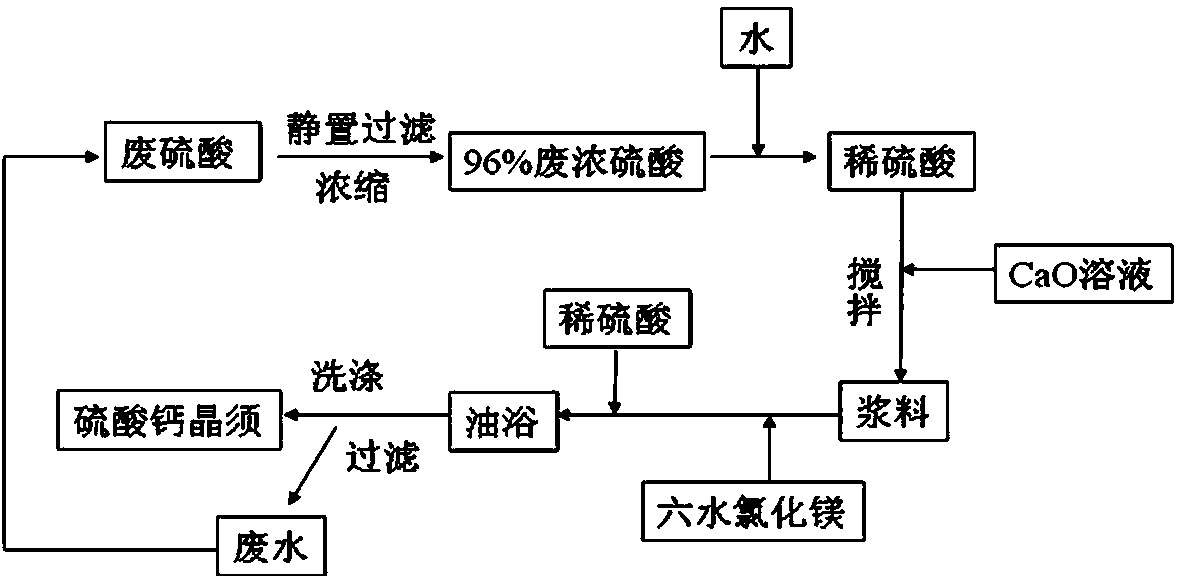
[0021] After the waste sulfuric acid was collected, it was left to settle for 3 days, and then filtered to remove particulate matter and other inorganic salt ions. After concentration, waste concentrated sulfuric acid with a concentration of 96% was obtained, and waste concentrated sulfuric acid was mixed with water to obtain 20% waste dilute sulfuric acid.
[0022] (1) Add 10 ml of 0.05mol / L calcium oxide solution into the flask, and add 10% dilute sulfuric acid at a ratio of 3:0.1 between calcium ions and sulfate ions, and stir for 30 min.
[0023] (2) Then add magnesium chloride hexahydrate according to the ratio of calcium ion to magnesium ion 40:1.
[0024] (3) Then add 10% dilute sulfuric acid at the ratio of calcium ion and sulfate ion at a ratio of 1:50, and react in an oil bath at 100 °C for 100 min.
[0025] (4) After the reaction, filter, dissolve the filter cake in water and let stand for 2 h, filter again, wash and dry to obtain calcium sulfate whiskers.
Embodiment 2
[0027] After the waste sulfuric acid was collected, it was left to settle for 2 days, and then filtered to remove particulate matter and other inorganic salt ions. After concentration, the waste concentrated sulfuric acid with a concentration of 96% was obtained, and the waste concentrated sulfuric acid was mixed with water to obtain 40% waste dilute sulfuric acid.
[0028] (1) Add 50 ml of 0.15mol / L calcium oxide solution into the flask, and add 40% dilute sulfuric acid at a ratio of 1:1 between calcium ions and sulfate ions, and stir for 100 min.
[0029] (2) Then add magnesium chloride hexahydrate according to the ratio of calcium ion to magnesium ion 30:1.
[0030] (3) Then add 40% dilute sulfuric acid at a ratio of 1:30 of calcium ions and sulfate ions, and react in an oil bath at 150°C for 200 minutes.
[0031] (4) After the reaction is completed, filter, dissolve the filter cake in water and let it stand for 10 hours, then filter again, wash and dry to obtain calcium su...
Embodiment 3
[0033] After the waste sulfuric acid was collected, it was left to settle for 1 day, and then filtered to remove particulate matter and other inorganic salt ions. After concentration, the waste concentrated sulfuric acid with a concentration of 96% was obtained, and the waste concentrated sulfuric acid was mixed with water to obtain 20% waste dilute sulfuric acid.
[0034] (1) Add 100 ml of 0.30mol / L calcium oxide solution to the flask, and add 75% dilute sulfuric acid at a ratio of 1:30 between calcium ions and sulfate ions, and stir for 300 min.
[0035] (2) Then add magnesium chloride hexahydrate according to the ratio of calcium ion to magnesium ion 20:1.
[0036] (3) Then add 75% dilute sulfuric acid at a ratio of calcium ion and sulfate ion at a ratio of 1:15, and react in an oil bath at 200°C for 360 minutes.
[0037] (4) After the reaction is completed, filter, dissolve the filter cake in water and let it stand for 18 hours, then filter again, wash and dry to obtain ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com