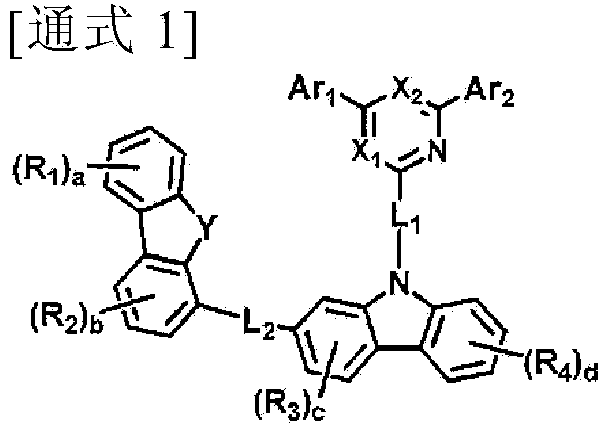
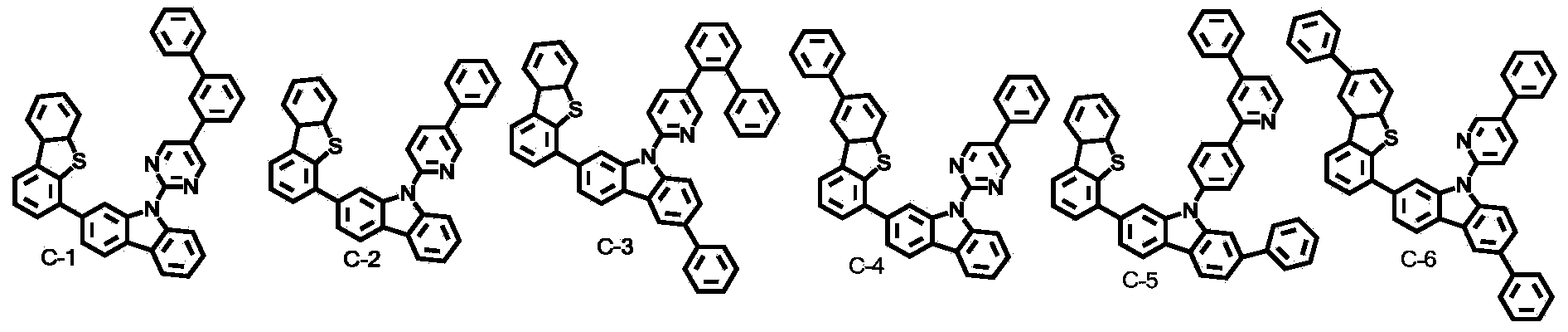
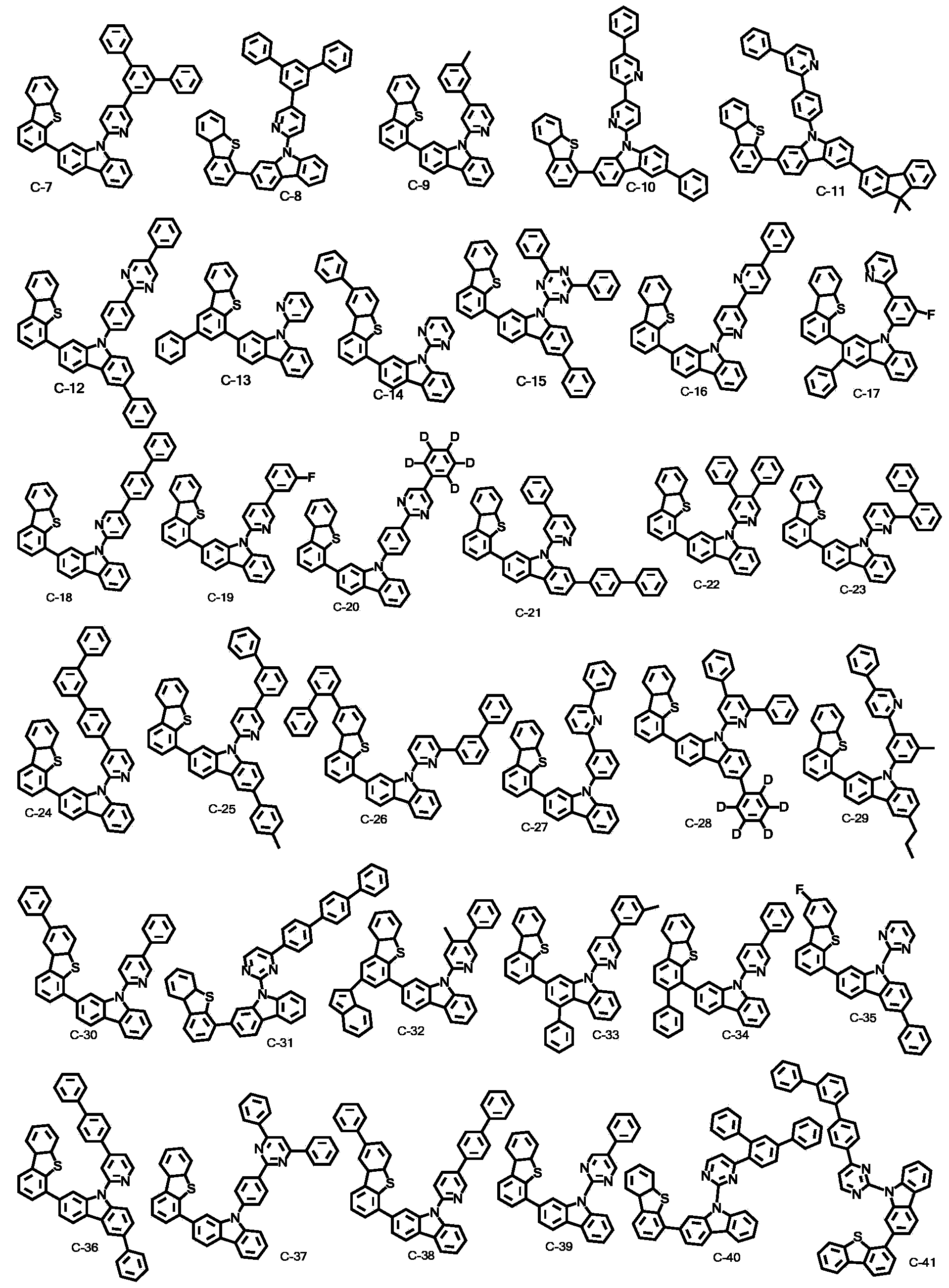
Novel organic electroluminescent compounds and an organic electroluminescent device using the same
A compound and luminescent technology, applied in organic chemistry, electrical solid devices, electrical components, etc., can solve the problems of unrevealed carbazole skeleton, decomposition, inability to provide luminous efficiency, driving voltage and working life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0063] [Preparation Example 1]: Preparation of Compound C-31
[0064]
[0065] Preparation of Compound A-2
[0066] 20g (0.087mol) of compound A-1, 49.6g (0.175mol) of 4-bromoiodobenzene, 3.039g (0.0026mol) of Pd(PPh 3 ) 4 , 1M Na 2 CO 3 and 400mL of toluene were mixed and stirred at reflux. After 3 hours, the mixture was cooled to room temperature, and distilled water was added thereto. The mixture was extracted with EA and MgSO 4 to dry. The resulting solid was distilled under reduced pressure and purified by column chromatography to obtain compound A-2 (23 g, 0.065 mol, 75.6%).
[0067] Preparation of Compound A-3
[0068] 23 g (0.065 mol) of compound A-2 was dissolved in 700 mL of THF. To the mixture was slowly added 39 mL of n-BuLi (0.098 mol, 2.5 M in hexane) at -78 °C. After 1 hour, 30.2 mL (0.131 mol) of triisopropyl borate was added thereto. After stirring at room temperature for 12 hours and adding distilled water to it, the mixture was extracted wit...
preparation Embodiment 2
[0078] [Preparation Example 2]: Preparation of Compound C-41
[0079]
[0080] Preparation of Compound 2-2
[0081] 25g (0.126mol) compound 2-1, 89.3g (0.316mol) 4-bromoiodobenzene, 2.66g (0.0038mol) PdCl 2 (PPh 3 ) 2 , 150mL2M Na 2 CO 3 , 150 mL of toluene and 30 mL of ethanol were mixed, stirred at 110° C. for 3 hours, and then distilled water was added thereto. The mixture was extracted with EA and MgSO 4 Dry and distill under reduced pressure. Purification was performed by column chromatography to obtain 31 g of compound 2-2 (0.100 mol, 80%).
[0082] Preparation of compound 2-3
[0083] 31 g (0.100 mol) of compound 2-2 was dissolved in 750 mL of THF, then 60 mL of n-BuLi (0.150 mol, 2.5M dissolved in hexane) was slowly added at -78°C. After 1 hour, 46 mL (0.200 mol) of triisopropyl borate was added to the mixture. The mixture was stirred at room temperature for 12 hours, and distilled water was added thereto. Then, the mixture was extracted with EA and M...
preparation Embodiment 3
[0089] [Preparation Example 3]: Preparation of Compound C-53
[0090]
[0091] Preparation of Compound C-53
[0092] Compound 3-1, 4-([1,1'-biphenyl]-4-yl)-2-chloro-6-phenylpyrimidine (5g, 14.58mmol) and compound A-5 (5.6g, 16.04 mmol) was dissolved in 100 mL DMF, then NaH (0.87 g, 60% in mineral oil, 21.87 mmol) was added thereto. The mixture was stirred at room temperature for 12 hours. After methanol was added, the mixture was filtered under reduced pressure. The obtained solid was purified by column chromatography to obtain compound C-53 (7 g, 10.67 mmol, 73.2%).
[0093] MS / FAB measured value 655.8; calculated value 655.21
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com