Sustained or controlled release solid composition comprising bupropion hydrochloride
A technology of bupropion hydrochloride and solid composition, which is applied in the field of sustained and controlled release solid composition, can solve the problems affecting the drug safety of patients, cumbersome preparation process, and long use time, and achieve controllable drug quality and safety, and simple preparation method , the effect of reducing the incidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0034] Drug-containing matrix sustained-release tablet core prescription (based on 1000 tablets)
[0035]
[0036]
[0037] Preparation Process:
[0038] Mix bupropion hydrochloride and hydroxypropyl methylcellulose K100M evenly, add PVPK30 aqueous solution as a binder to make a soft material, granulate with 18 mesh, control the drying temperature at 50°C, and keep the moisture content of the granules within 3.0%. 24-mesh granules, mixed with magnesium stearate, and compressed into tablets.
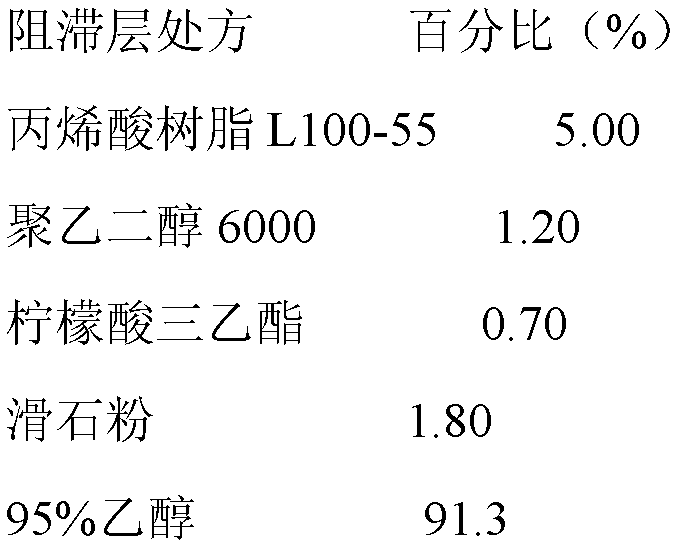
[0039] Add acrylic resin L100-55, polyethylene glycol 6000, triethyl citrate, and talcum powder to 95% ethanol in sequence, and after stirring evenly, use a coating pan for coating, and the weight gain of the coating is 6%-7% .
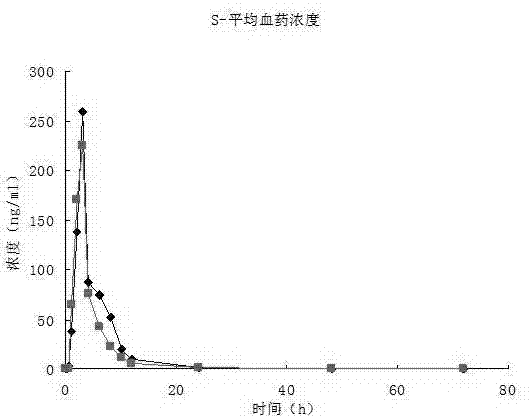
[0040] The present invention measures the release rate of the above-mentioned bupropion hydrochloride sustained-release tablet.
[0041] Release measurement conditions:...
Embodiment 2
[0062] Example 2 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0063] Drug-containing matrix sustained-release tablet core prescription (based on 1000 tablets)
[0064]
[0065] Preparation Process:
[0066] Mix bupropion hydrochloride and ethyl cellulose evenly, add 60% ethanol to make soft material, granulate with 18 mesh, control the drying temperature at 50°C, keep the water content of the granules within 3.0%, granulate with 24 mesh, add hard Magnesium fatty acid is mixed evenly and pressed into tablets.
[0067] Add acrylic resin L100-55, polyethylene glycol 6000, triethyl citrate, and talcum powder to 95% ethanol in sequence, and after stirring evenly, use a coating pan for coating, and the weight gain of the coating is 5.5%-6.5% .
[0068] The present invention measures the release rate of the above-mentioned bupropion hydrochloride sustained-release tablet.
[0069] Release measurement conditions:
[0070] Instrument: ZRS-8G, Tianjin Tian...
Embodiment 3
[0077] Example 3 Preparation of Bupropion Hydrochloride Sustained Release Tablets
[0078] Drug-containing matrix sustained-release tablet core prescription (based on 1000 tablets)
[0079]
[0080]
[0081] Preparation Process:
[0082] Mix bupropion hydrochloride and acrylic resin L100-55 evenly, add soft materials made of 80% ethanol, granulate with 18 mesh, control the drying temperature at 50°C, keep the water content of the granules within 3.0%, granulate with 24 mesh, add Magnesium stearate is mixed evenly and compressed into tablets.
[0083] Add ethyl cellulose, hypromellose, diethyl phthalate, and talcum powder to 90% ethanol in sequence, and after stirring evenly, use a coating pan to coat, and the weight gain of the coating is 4%-5 %.
[0084] The present invention measures the release rate of the above-mentioned bupropion hydrochloride sustained-release tablet.
[0085] Release measurement conditions:
[0086] Instrument: ZRS-8G, Tianjin Tianda Tianfa ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com