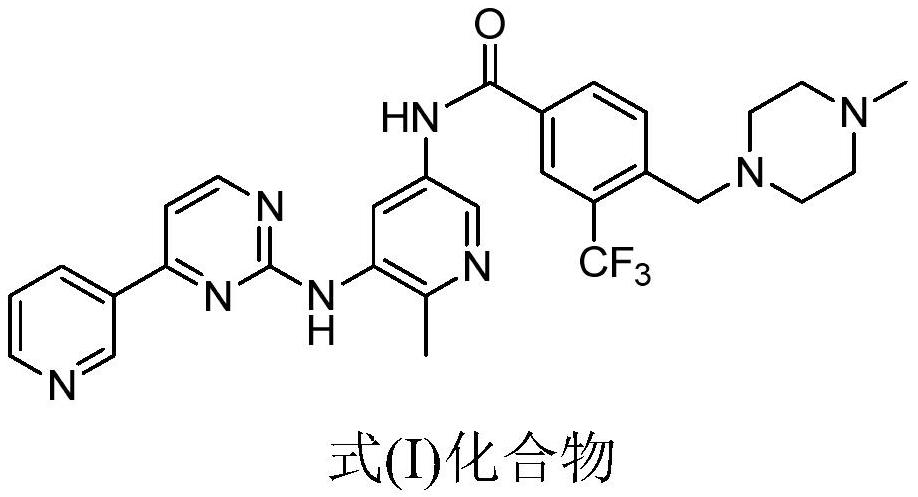
Pharmaceutical composition of aminopyrimidine compound and preparation method thereof
A composition and drug technology, which are applied in the field of pharmaceutical compositions of aminopyrimidine compounds and their preparation, can solve the problem that tyrosine kinase inhibitors cannot radically cure CML, cannot successfully treat CML patients, and reduce imatinib sensitivity, etc. To achieve the effect of improving bioavailability, controllable quality and safety, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
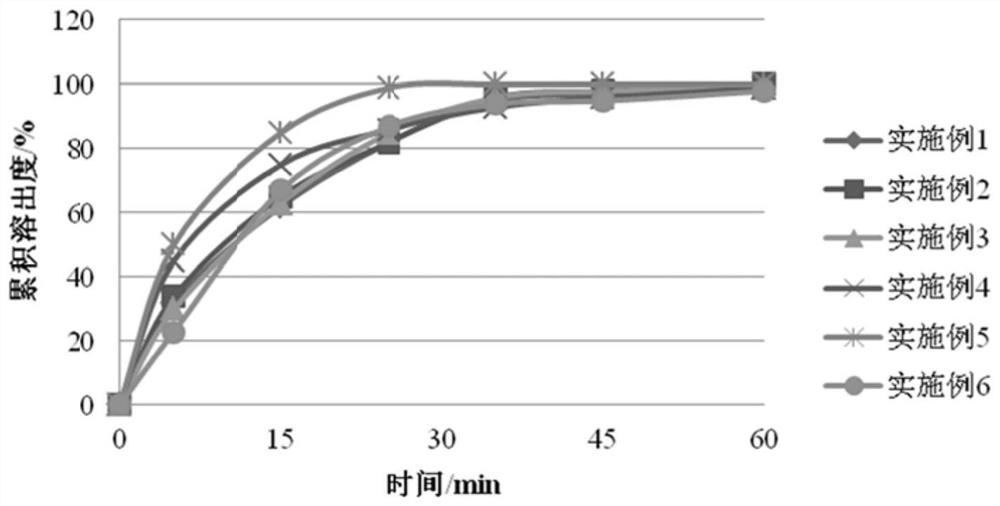
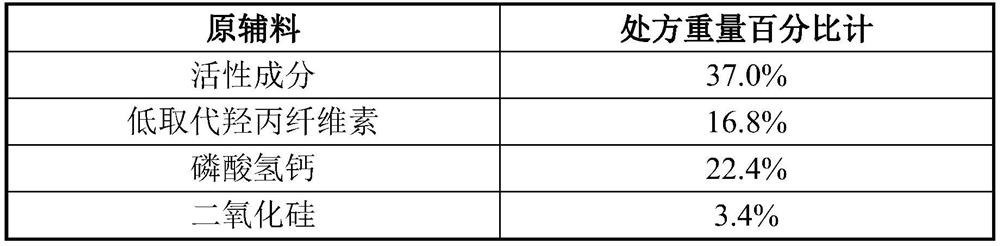
[0082] Example 1 50mg / 100mg / 300mg / 400mg tablet
[0083] Raw materials Prescription Weight Percentage Meter active ingredient 40.0% Low-substituted hydroxypropyl cellulose 20.0% Calcium hydrogen phosphate 12.0% silica 4.0% Carboxymethyl Starch Sodium 4.0% Magnesium stearate 1.0% 50% ethanol in water 19.0%
[0084] Pass the active ingredients, fillers, and disintegrants through a 60-mesh sieve, respectively weigh the prescribed amount of active ingredients, fillers, disintegrators, and lubricants, mix them evenly, and add an appropriate amount of wetting agent to prepare a suitable soft material. , 24-mesh granulation, drying at 60°C, 30-mesh granulation, then adding the prescribed amount of lubricant, mixing evenly, testing the content of intermediates, determining the weight of the tablet, pressing the tablet, and putting the core into a high-efficiency coating machine. Set the air inlet temperature at 60°C ± 5°C, contr...
Embodiment 2
[0085] Example 2 50mg / 100mg / 200mg / 300mg tablet
[0086] Raw materials Prescription Weight Percentage Meter active ingredient 39.8% Low-substituted hydroxypropyl cellulose 19.9% Calcium hydrogen phosphate 11.9% silica 4.0% Carboxymethyl Starch Sodium 3.8% Magnesium stearate 0.8% 50% ethanol in water 19.8%
[0087] Pass the active ingredient, filler, and disintegrating agent through a 60-mesh sieve, weigh the prescribed amount of the active ingredient, filler, disintegrating agent, and lubricant, add them to the wet granulator, mix evenly, and add an appropriate amount of lubricant. Wet agent, prepare suitable wet granules, transfer them to the boiling dryer, set the air inlet temperature to 60°C, wait until the moisture content is ≤6%, discharge and granulate, then add the prescribed amount of lubricant, mix evenly, Test the content of intermediates, determine the weight of the tablet, press the tablet, put the core into...
Embodiment 3
[0088] Example 3 50mg / 100mg / 200mg / 300mg tablet
[0089] Raw materials Prescription Weight Percentage Meter active ingredient 42.0% Low-substituted hydroxypropyl cellulose 19.0% Calcium hydrogen phosphate 11.0% silica 4.0% Carboxymethyl Starch Sodium 4.0% Magnesium stearate 1.0% 50% ethanol in water 19.0%
[0090] Pass the active ingredient, filler, and disintegrating agent through a 60-mesh sieve, weigh the prescribed amount of the active ingredient, filler, disintegrating agent, and lubricant, add them to the wet granulator, mix evenly, and add an appropriate amount of lubricant. Wet agent, prepare suitable wet granules, transfer them to the boiling dryer, set the air inlet temperature to 60°C, wait until the moisture content is ≤6%, discharge and granulate, then add the prescribed amount of lubricant, mix evenly, Test the content of intermediates, determine the weight of the tablet, press the tablet, put the core into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com