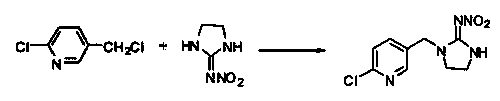Synthesis method of imidacloprid as insecticide
A synthetic method, imidacloprid technology, applied in the field of synthesis of the insecticide imidacloprid, can solve the problems of low cost of raw materials, pollution of "three wastes", long steps, etc., and achieve the effect of high yield and short synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] A kind of synthetic method of insecticide imidacloprid is characterized in that, comprises the steps:
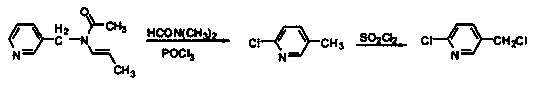
[0016] 1) Using 280g of N-benzyl-N-propenylacetamide as raw material, in the solvent dimethylformamide, react with 919.8g of phosphorus oxychloride at 100°C for 14-16h with stirring, and then further dichloride Chlorination in sulfone, lowering the temperature and maintaining the temperature at 15-20°C, performing vacuum suction to remove unreacted phosphorus oxychloride to obtain 291.7g of 2-chloro-5-chloromethylpyridine;
[0017] 2) Dissolve 291.7g 2-chloro-5-chloromethylpyridine and 257.4g imidazolidine in acetonitrile, add K 2 CO 3 , with the participation of CsCl, heated to reflux for 5 hours to obtain 434.69g of the product 1-(6-chloro-3-pyridylmethyl)-N-nitroimidazole-2-imine. The product yield is 94.4%.
Embodiment 2
[0019] A kind of synthetic method of insecticide imidacloprid is characterized in that, comprises the steps:
[0020] 1) Using 280g of N-benzyl-N-propenylacetamide as the raw material, in the solvent dimethylformamide, react with 1839.6g of phosphorus oxychloride at 100°C for 14-16h with stirring, and then further dichloride Chlorination in sulfone, lowering the temperature and maintaining the temperature at 15-20°C, performing vacuum suction to remove unreacted phosphorus oxychloride to obtain 307.8g of 2-chloro-5-chloromethylpyridine;
[0021] 2) Dissolve 307.8g 2-chloro-5-chloromethylpyridine and 296.4g imidazolidine in acetonitrile, add K 2 CO 3 , with the participation of CsCl, heated to reflux for 5 hours to obtain 473.05 g of the product 1-(6-chloro-3-pyridylmethyl)-N-nitroimidazole-2-imine. The product yield is 97.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com