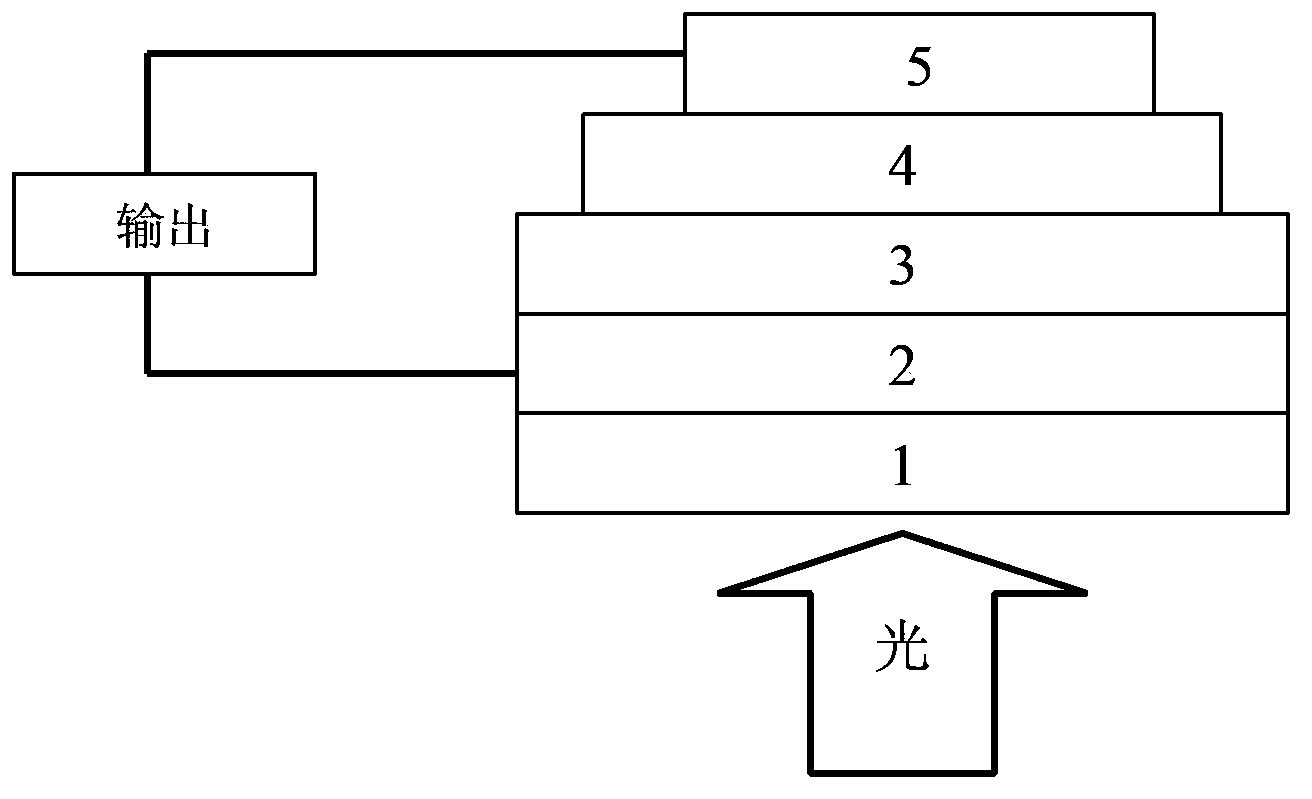
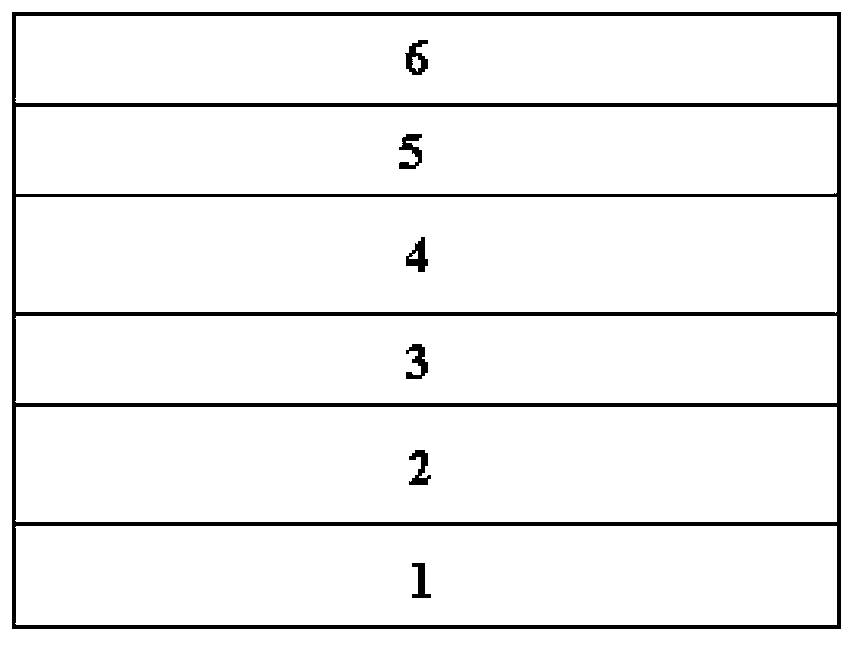
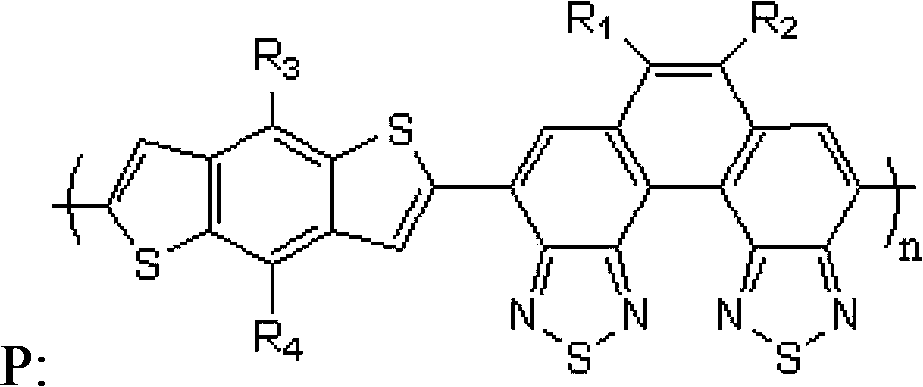
Benzodithiophene-benzodi(benzothiadiazole) containing copolymer, preparation and application thereof
A technology of benzothiadiazole and benzodithiophene is applied in the fields of benzodithiophene-benzodipolymer and its preparation and application, which can solve problems such as low energy conversion efficiency, achieve good thermal stability, The effect of good electron transport properties and good film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] A benzodithiophene-benzobis(benzothiadiazole) copolymer, namely poly{4,8-(dioctyl)oxy-benzo[1,2-b:4,5-b ']dithiophene-6,7-bis(3,7-dimethyloctyl)-benzo[2,1-e:3,4-e]bis(benzothiadiazole)} (n=55 ), denoted as copolymer P1, the general formula is as follows:
[0063]
[0064] The preparation method comprises the following steps:
[0065] (1), 4,9-dibromo-6,7-bis(3,7-dimethyloctyl)-benzo[2,1-e:3,4-e]bis(benzothiadiazole ) (B1) preparation method comprises the following steps:
[0066] (1) Preparation of compound 5-nitro-2,1,3 benzothiadiazole:
[0067]
[0068] Add 4-nitrobenzene-1,2-diamine (22.95g, 0.15mol) and 100mL thionyl chloride (SOCl 2 ), stirred and slowly added 2 mL of pyridine dropwise. At room temperature, 2-amino-5-nitroaniline was not completely dissolved, and the solution was orange. After heating, reflux at 80~90°C for 24 hours, stop the reaction, and heat to 80°C. Distill off excess thionyl chloride (SOCl 2 ), the reaction product was cooled to r...
Embodiment 2
[0090] A benzodithiophene-benzobis(benzothiadiazole) copolymer, poly{4,8-dioctyl-benzo[1,2-b:4,5-b']dithiophene- 6,7-bis(3,7-dimethyloctyl)-benzo[2,1-e:3,4-e]bis(benzothiadiazole)} (n=50), recorded as copolymerization Thing P2, general formula is as follows:
[0091]
[0092] The preparation method comprises the following steps:
[0093] (1) The preparation method of 4,8-dioctyl-2,7-di(tributyltinyl)-benzo[1,2-b:4,5-b']dithiophene (A2) is as follows:
[0094] Provide a compound C1 with a structural formula as shown in formula C1, namely 4,8-dioctyl-2,7-dibromo-benzo[1,2-b:4,5-b']dithiophene;
[0095] Compound C1 (11.46g, 0.02mol) and 100mL THF were added to a round bottom flask, and under nitrogen protection, the temperature was lowered to -78°C, and 16.8mL (2.5M, 0.04mol) of n-butyllithium in n-hexane was added dropwise, React for 1 hour, add 11.2mL tributyltin chloride (SnBu 3 Cl) (0.04mol), the temperature was raised to room temperature after 1 hour of reaction, and ...
Embodiment 3
[0102] A benzodithiophene-benzobis(benzothiadiazole) copolymer, that is, poly{4,8-bis(5'-hexadecyl-2'-thienyl)-benzo[1, 2-b:4,5-b']dithiophene-benzo[1,2-b:4,5-b']dithiophene-6,7-di(3,7-dimethyloctyl)- Benzo[2,1-e:3,4-e]bis(benzothiadiazole)} (n=85), denoted as copolymer P3, the general formula is as follows:
[0103]
[0104] The preparation method comprises the following steps:
[0105] (1), 4,8-bis(5'-hexadecyl-2'-thienyl)-2,7-bis(tributyltinyl)-benzo[1,2-b:4,5-b '] Dithiophene (A3) was prepared as follows:
[0106] Provide a compound C2 with a structural formula as shown in formula C2, namely 4,8-bis(5'-hexadecyl-2'-thienyl)-2,7-dibromo-benzo[1,2-b:4 ,5-b']dithiophene;
[0107] Add compound C2 (9.61g, 0.01mol) and 100mL THF into a round bottom flask, under nitrogen protection, cool down to -78°C, add dropwise 8.4mL (2.5M, 0.02mol) of n-butyllithium in n-hexane, React for 1 hour, add SnBu 3 Cl 5.60mL (0.02mol), reacted for 1 hour, then warmed up to room temperature,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com