Traditional Chinese medicinal composition for treating dysmenorrhea and preparation method thereof
A composition and technology of traditional Chinese medicine are applied in the field of traditional Chinese medicine composition for treating primary dysmenorrhea and the field of preparation thereof, which can solve the problems of inability to satisfy patients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of pharmaceutical preparation
[0032] 1. Formulation
[0033] Angelica (16kg) 16 parts Chuanxiong (14kg) 14 parts Sanqi (8kg) 8 parts
[0034] Motherwort (10kg) 10 parts Peach kernel (5kg) 5 parts Tulip (6kg) 6 parts
[0035] 2. adopt the preparation method provided by the invention to prepare pharmaceutical preparations
[0036] details as follows:
[0037] 1) Take 16 kg of Angelica sinensis and 8 kg of Panax notoginseng and grind them into the coarsest powder, use 80% ethanol as solvent, soak for 24 hours, slowly percolate, collect the initial percolation liquid, and store it in another container; continue percolation until the percolation liquid is nearly Until it is colorless or light yellow, collect the continuous filtrate, combine the filtrate for later use, and collect the filter residue in another device;
[0038] 2) Take 14 kg of Ligusticum chuanxiong and 6 kg of tulips and crush them into the coarsest powder, use ...
Embodiment 2
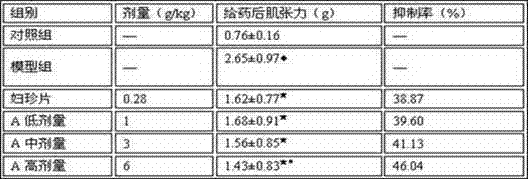
[0041] Embodiment 2: The influence of the pharmaceutical preparation of the present invention on the thermal stimulation pain threshold of mice
[0042] 1. Test material
[0043] The drug preparation A prepared in Example 1 of the present invention has a content of 12 g crude drug / bag; Fuzhen Tablets, batch number 2120213, provided by Guangxi Hefeng Pharmaceutical Co., Ltd.
[0044] , experimental animals
[0045] Kunming mice, weighing 20±2g.
[0047]This experiment adopts intragastric administration, the control group: the same volume of water is given by intragastric administration; the drug preparation A is set up as high, medium and low dosage groups, and the doses are 6g crude drug / kg body weight, 3 crude drug / kg body weight, 1g crude drug / kg body weight; the positive control group is the Fuzhen tablet group: each tablet of Fuzhen tablets weighs 0.26g, and the daily dose is 10 tablets, which is equivalent to 0.39g patent medicine...
Embodiment 3
[0055] Embodiment 3: the influence of the pharmaceutical preparation of the present invention on mice's acetic acid writhing response
[0056] 1. Test material
[0057] The drug preparation A prepared in Example 1 of the present invention has a content of 12 g crude drug / bag; Fuzhen Tablets, batch number 2120213, provided by Guangxi Hefeng Pharmaceutical Co., Ltd.
[0058] , experimental animals
[0059] Kunming mice, weighing 20±2g.
[0060] , test drug dose
[0061] This experiment adopts intragastric administration, the control group: the same volume of water is given by intragastric administration; the drug preparation A is set up as high, medium and low dosage groups, and the doses are 6g crude drug / kg body weight, 3g crude drug / kg body weight, 1g crude drug / kg body weight; the positive control group is the Fuzhen tablet group: each tablet of Fuzhen tablets weighs 0.26g, and the daily dose is 10 tablets, which is equivalent to 0.39g patent medicine / kg body...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com