Preparation method for nuclear-grade high-purity boric acid
A high-purity boric acid, nuclear-grade technology, applied in the direction of boron oxides, etc., can solve the problems of unfavorable nuclear power industry development, high product cost, environmental impact, etc., and achieve the effect of easy operation, low production cost and thorough iron removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
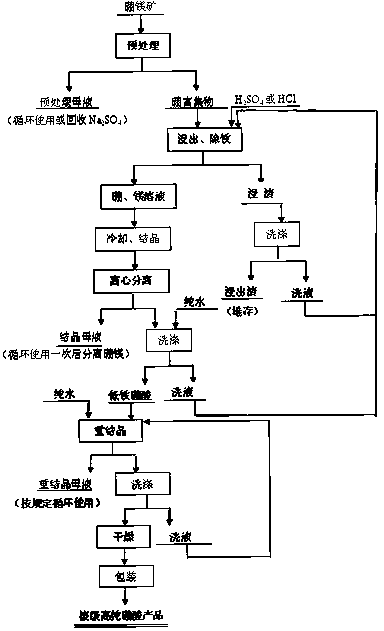
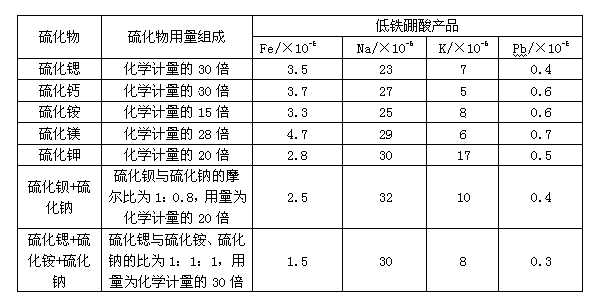
[0032] A method for preparing nuclear-grade high-purity boric acid is characterized in that it includes the following process steps: 1) leaching boron-magnesium ore raw materials with water to remove water-soluble impurities sodium and potassium to obtain Na 2 Low-sodium boron-magnesite with O content <0.6%; 2) The low-sodium boron-magnesite is further leached with sulfuric acid or hydrochloric acid, and after deep iron removal with compound additives, it is filtered, cooled and crystallized, solid-liquid separated and Washing to obtain boric acid with low iron and sodium content; 3) Dissolving boric acid with low iron and sodium content in pure water, recrystallization, dehydration, and drying to obtain nuclear-grade high-purity boric acid products.
[0033] The composite additive is a combination of sulfide and ammonia water, which is used after dissolving the sulfide in ammonia water or mixing the sulfide with ammonia water.
[0034] The sulfide is any one of ammonium sulfi...
Embodiment 2
[0047] A method for preparing nuclear-grade high-purity boric acid is characterized in that it includes the following process steps: 1) leaching boron-magnesium ore raw materials with water to remove water-soluble impurities sodium and potassium to obtain Na 2 Low-sodium boron-magnesite with O content <0.6%; 2) The low-sodium boron-magnesite is further leached with sulfuric acid or hydrochloric acid, and after deep iron removal with compound additives, it is filtered, cooled and crystallized, solid-liquid separated and Washing to obtain boric acid with low iron and sodium content; 3) Dissolving boric acid with low iron and sodium content in pure water, recrystallization, dehydration, and drying to obtain nuclear-grade high-purity boric acid products.
[0048] The composite additive is a combination of sulfide and ammonia water, which is used after dissolving the sulfide in ammonia water or mixing the sulfide with ammonia water.
[0049] The sulfide is a combination of any two ...
Embodiment 3
[0061] A method for preparing nuclear-grade high-purity boric acid is characterized in that it includes the following process steps: 1) leaching boron-magnesium ore raw materials with water to remove water-soluble impurities sodium and potassium to obtain Na 2 Low-sodium boron-magnesite with O content <0.6%; 2) The low-sodium boron-magnesite is further leached with sulfuric acid or hydrochloric acid, and after deep iron removal with compound additives, it is filtered, cooled and crystallized, solid-liquid separated and Washing to obtain boric acid with low iron and sodium content; 3) Dissolving boric acid with low iron and sodium content in pure water, recrystallization, dehydration, and drying to obtain nuclear-grade high-purity boric acid products.
[0062] The composite additive is a combination of sulfide and ammonia water, which is used after dissolving the sulfide in ammonia water or mixing the sulfide with ammonia water.
[0063] The sulfide is a combination of any thre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com