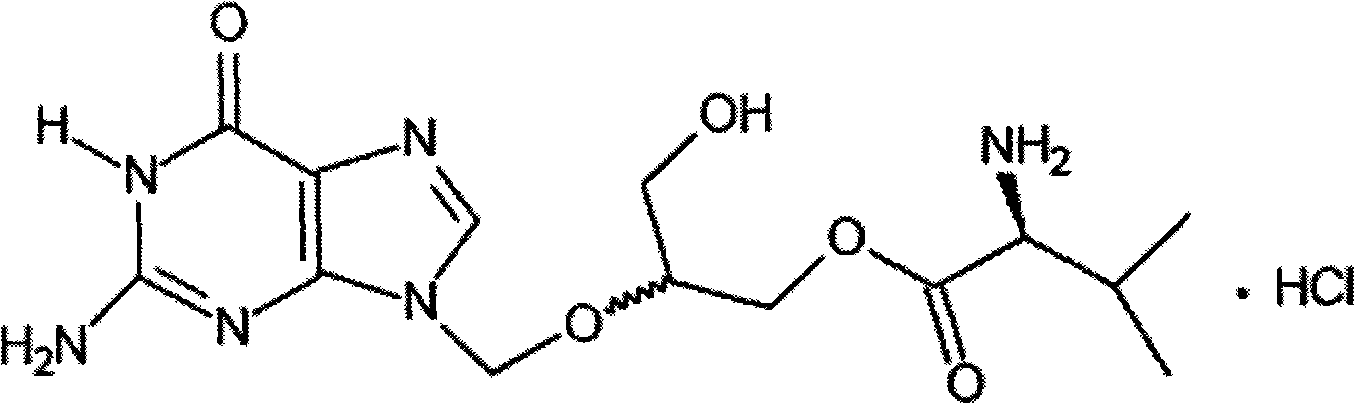
Synthesis method of valganciclovir as hydrochloric acid
A kind of valganciclovir hydrochloride, the technology of synthetic method, is applied in the preparation field of antiviral drug, can solve the problem such as active reagent is not easy to obtain, synthetic route is long, achieves few synthetic steps, safety improves, and is suitable for industrialized production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
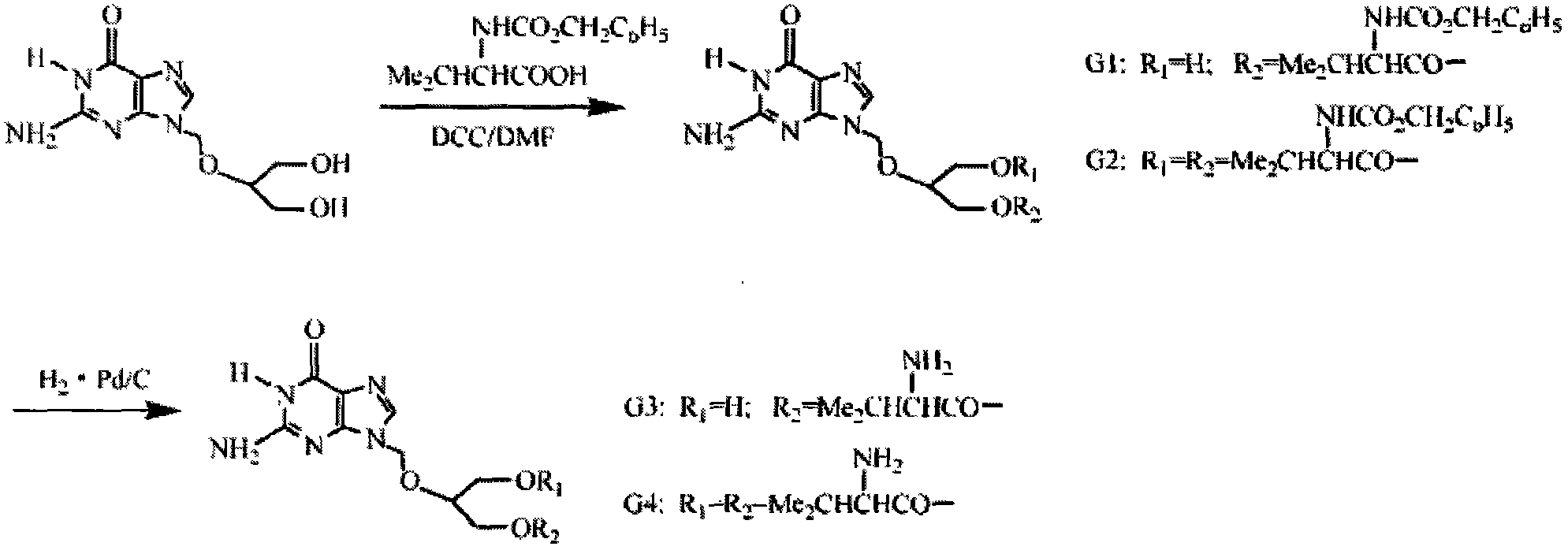
[0025] The synthetic technique of embodiment valganciclovir hydrochloride
[0026] (1) Preparation of N-benzyloxycarbonyl-valine (Cbz-Va)
[0027] Dissolve L-valine in sodium hydroxide solution, cool in an ice-salt bath under magnetic stirring, keep the internal temperature not higher than 10°C, add benzyloxychloromethyl ester dropwise to the above solution, and continue cooling in an ice-water bath after adding Stirred under low temperature for 2h, then stirred at room temperature for 6h, and TLC checked that the starting material disappeared. The basic reaction mixture was extracted with chloroform to remove excess benzyloxychloromethyl ester or its decomposed benzyl alcohol. The aqueous layer was neutralized with hydrochloric acid to pH = 2 under cooling in an ice-water bath, extracted with CHC13, washed with water, dried over anhydrous Na2SO4, and evaporated to dryness to obtain the product.
[0028] (2) 2-[(2-amino-1,6-dihydro-6-oxo-purine-9-)methoxy]-3-hydroxyl-1-propy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com