Dithienothiophene-naphthyl tetracarboxylic diimide conjugated polymer and preparation method and application thereof
A naphthalene tetracarboxylic acid diimide, conjugated polymer technology, applied in the field of solar cell devices and organic electroluminescent devices, dithienothiophene-naphthalene tetracarboxylic acid diimide conjugated polymer, can Solve the problem of low carrier mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
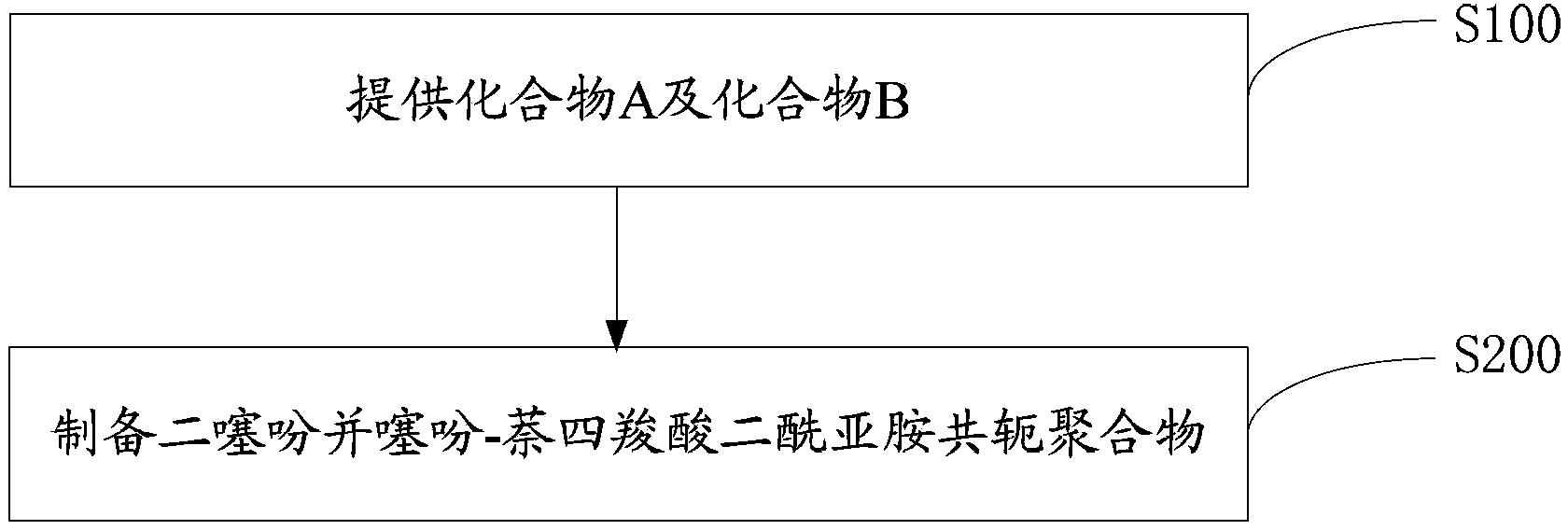
[0062] The preparation method of the dithienothiophene-naphthalene tetracarboxylic acid diimide conjugated polymer of one embodiment, such as figure 1 shown, including the following steps:
[0063] Step S100, providing compound A and compound B represented by the following structural formula.
[0064] A is: B is:
[0065] Among them, R 1 for C 1 ~C 20 Alkyl groups or aromatic groups with the following structure:
[0066]
[0067] R 2 , R 3 , R 4 for H, C 1 ~C 20 Alkyl or C 1 ~C 20 of alkoxy.
[0068] Wherein the preparation of compound A comprises the following steps:
[0069] To a solution of 2,6-dibromo-dithieno[3,2-b:2',3'-d]thiophene in tetrahydrofuran at -78°C, add n-butyllithium, The molar ratio of [3,2-b:2',3'-d]thiophene to n-butyllithium is 1:3, after warming up to room temperature, stirring and reacting for 0.5 hours, cooling to -78°C and adding trimethyl chloride Tin (Me 3 SnCl) reaction, heated to room temperature and reacted for 24 hours to ...
Embodiment 1
[0100] This embodiment discloses a dithienothiophene-naphthalene tetracarboxylic acid diimide conjugated polymer with the following structure:
[0101]
[0102] n=39.
[0103] The preparation steps of above-mentioned dithienothiophene-naphthalene tetracarboxylic acid diimide conjugated polymer are as follows:
[0104] 1. Synthesis of compound 2,6-bistrimethyltin-dithieno[3,2-b:2',3'-d]thiophene:
[0105]
[0106] t-BuLi (5.3mL, 1.4mol / L, 7.5mmol) was added dropwise to 2,6-dibromo-dithieno[3,2-b:2',3'-d at -78°C ] Thiophene (2.5mmol, 0.885g) in tetrahydrofuran solution (100mL). The mixture was slowly returned to room temperature and stirred for 0.5 h. Then cooled to -78°C, trimethyltin chloride (7.5mmol, 7.5mL) was added dropwise to the above solution. Slowly return to room temperature and stir overnight. The above reaction solution was quenched with water, tetrahydrofuran was removed by rotary evaporation, extracted with chloroform / water, washed with water, and drie...
Embodiment 2
[0117] This embodiment discloses a dithienothiophene-naphthalene tetracarboxylic acid diimide conjugated polymer with the following structure:
[0118]
[0119] n=73.
[0120] The preparation steps of above-mentioned dithienothiophene-naphthalene tetracarboxylic acid diimide conjugated polymer are as follows:
[0121] 1. The synthesis of the compound 2,6-bistrimethyltin-dithieno[3,2-b:2',3'-d]thiophene is the same as in Example 1.
[0122] 2. Preparation of N,N'-di-(n-eicosyl)-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic acid diimide:
[0123]
[0124] Under nitrogen, n-eicosylamine (0.30 g, 1 mmol) was added to propionic acid ( 15mL) solution, refluxed overnight. After cooling to room temperature, the reaction solution was poured into aqueous sodium hydroxide solution and extracted with chloroform. The organic solvent was removed, washed with ethyl acetate, dissolved in chloroform, and then column chromatographed (alumina column). Removal of solvent gave a solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com