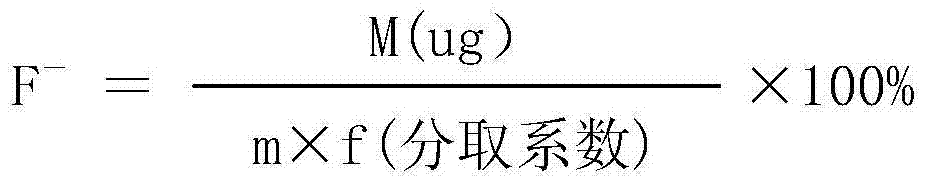
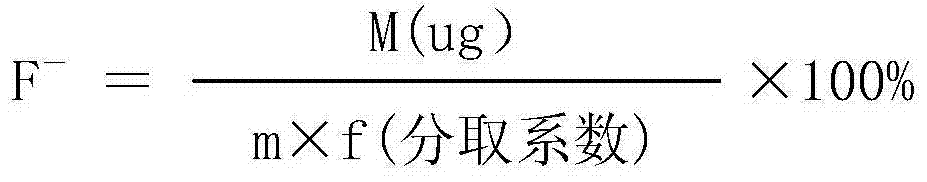
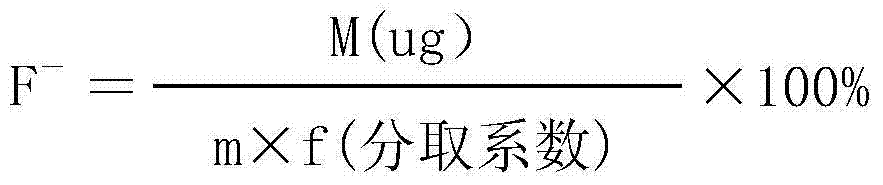
Analytical method for directly measuring fluorine ion content in carbonic acid rare earth by using fluorine ion electrode method
An analysis method and fluoride ion technology, applied in the direction of electrochemical variables of materials, etc., can solve the problems of energy consumption, high analysis cost, inability to meet accurate analysis data, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Determination process of fluoride ion content in mixed rare earth carbonate:
[0066] 1) Weigh 1.000g of the mixed rare earth carbonate sample and add 10ml of hydrochloric acid with a volume ratio of 1:1 into the beaker. After completely dissolving, transfer it into a 200ml volumetric flask to obtain solution A.
[0067] 2) Take 5ml of solution A and place it in a 50ml volumetric flask, add p-nitrophenol indicator, add ammonia water with a volume ratio of 1:4 to turn yellow, add hydrochloric acid with a volume ratio of 1:4 to become colorless, and adjust the acidity to pH=6 -6.5, then add 10ml of total ionic strength buffer solution prepared by sodium citrate and sodium nitrate, add 2ml of 5% sodium acetate in the mass ratio and adjust the volume to the mark to obtain solution B.
[0068] 3) Connect the fluoride ion selective electrode and the calomel electrode in parallel to the acidity meter, insert it into a plastic beaker filled with pure water, add a stirring bar, ...
Embodiment 2
[0079] Determination process of fluoride ion content in lanthanum carbonate
[0080] 1) Weigh 2.000g of lanthanum carbonate sample and add 10ml of hydrochloric acid with a volume ratio of 1:1 into the beaker, completely dissolve it and transfer it into a 200ml volumetric flask to obtain solution A,
[0081] 2) Take 5ml of solution A and place it in a 50ml volumetric flask, add p-nitrophenol indicator, add ammonia water with a volume ratio of 1:4 to turn yellow, add hydrochloric acid with a volume ratio of 1:4 to become colorless, and adjust the acidity to pH=6 -6.5, then add 10ml of total ionic strength buffer solution prepared by sodium citrate and sodium nitrate, and then add 2ml of 5% sodium acetate in mass ratio to constant volume to the mark to obtain solution B.
[0082] 3) Connect the fluoride ion selective electrode and the calomel electrode in parallel to the acidity meter, insert it into a plastic beaker filled with pure water, add a stirring bar, stir on a magnetic ...
Embodiment 3
[0093] Determination process of fluoride ion content in praseodymium and neodymium carbonate
[0094] 1) Weigh 3.000g of praseodymium and neodymium carbonate sample and add 10ml of hydrochloric acid with a volume ratio of 1:1 into the beaker, completely dissolve it and transfer it into a 200ml volumetric flask to obtain solution A,
[0095] 2) Take 5ml of solution A and place it in a 50ml volumetric flask, add p-nitrophenol indicator, add ammonia water with a volume ratio of 1:4 to turn yellow, add hydrochloric acid with a volume ratio of 1:4 to become colorless, and adjust the acidity to pH=6 -6.5, then add 10ml of total ionic strength buffer solution prepared by sodium citrate and sodium nitrate, and then add 2ml of 5% sodium acetate in mass ratio to constant volume to the mark to obtain solution B.
[0096] 3) Connect the fluoride ion selective electrode and the calomel electrode in parallel to the acidity meter, insert it into a plastic beaker filled with pure water, add a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com