Pulsatile delivery composition for treating diabetes mellitus and preparation method thereof
A technology of pulse and mixture, which is applied in the direction of drug combination, drug formulation, drug delivery, etc., which can solve the problems of poor stability, short biological half-life, aggravating the physical and psychological burden of patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
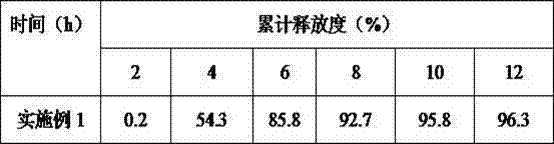
[0015] The formula of liraglutide combined with insulin for the treatment of type Ⅱ diabetes mellitus pulse controlled release tablets consists of: accurately weigh 400mg liraglutide, 250mg insulin, 100mg sodium carboxymethyl starch, 100mg talcum powder, 800mg ethyl cellulose, 100mg propylene glycol, Polyethylene glycol 100mg, sodium lauryl sulfate 50mg, sucrose 50mg, 200ml 75% ethanol (finally removed). Prepare 1000 pulse controlled-release tablets according to the formula.
[0016] Its preparation process is as follows: (1) Preparation of tablet core: dissolve liraglutide, insulin, sodium lauryl sulfonate, sucrose, and sodium carboxymethyl starch in the formula in soft material made of 75% ethanol, pass through 24 mesh Sieve and granulate, pass through a 20-mesh sieve after drying, add talcum powder, mix evenly, and press into tablets; (2) Preparation of pulse-controlled release tablets: half the amount of ethyl cellulose, propylene glycol, and polyethylene glycol compositio...
Embodiment 2
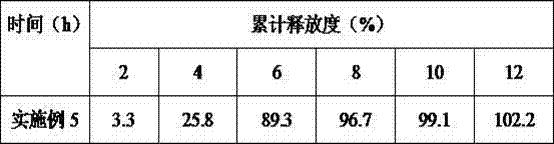
[0027]Liraglutide combined with insulin in the treatment of type Ⅱ diabetes pulse controlled-release tablets 1000 tablets The formula is composed of: accurately weighed 550mg liraglutide, 250mg insulin, 100mg hypromellose, 120mg talcum powder, 500mg hypromellose, glycerin Triacetate 80mg, polyethylene glycol 100mg, sodium dodecyl sulfonate 50mg, lactose 100mg, 200ml 75% ethanol (finally removed). Prepare 1000 pulse controlled-release tablets according to the formula.
[0028] Its preparation process is as follows: (1) Preparation of tablet core: dissolve liraglutide, insulin, sodium lauryl sulfonate, lactose, and hydroxypropyl cellulose in the formula in 75% ethanol soft material, pass through a 24-mesh sieve Granulate, pass through a 20-mesh sieve after drying, add talcum powder, mix evenly, and compress into tablets; (2) Preparation of pulse-controlled release tablets: a combination of half-dose hypromellose, triacetin, and polyethylene glycol The material is placed in the ...
Embodiment 3
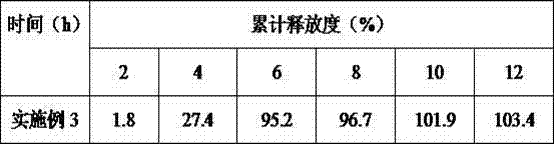
[0040] Liraglutide Combined with Insulin for Treatment of Type Ⅱ Diabetes Pulse Controlled Release Tablets 1000 Tablets The formula consists of: accurately weigh 600mg liraglutide, 300mg insulin, 150mg sodium carboxymethyl starch, 120mg magnesium stearate, phthalic acid Cellulose 400mg, dibutyl phthalate 80mg, povidone 100mg, dioctyl sodium sulfosuccinate 100mg, starch 150mg, 200ml 75% ethanol (finally removed). Prepare 1000 pulse controlled-release tablets according to the formula.
[0041] The preparation process is as follows: (1) Preparation of tablet cores: dissolve liraglutide, insulin, sodium dioctyl succinate sulfonate, starch, and sodium carboxymethyl starch in the formula in soft materials made of 75% ethanol, and pass 24 Mesh sieve granulation, after drying, pass through a 20-mesh sieve for granulation, add talcum powder, mix evenly, and tablet; , the povidone composition is placed in the die, and then the tablet core is placed at the center of the die on the coat ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com