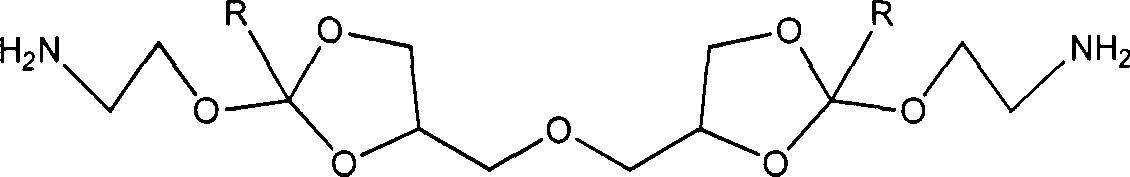
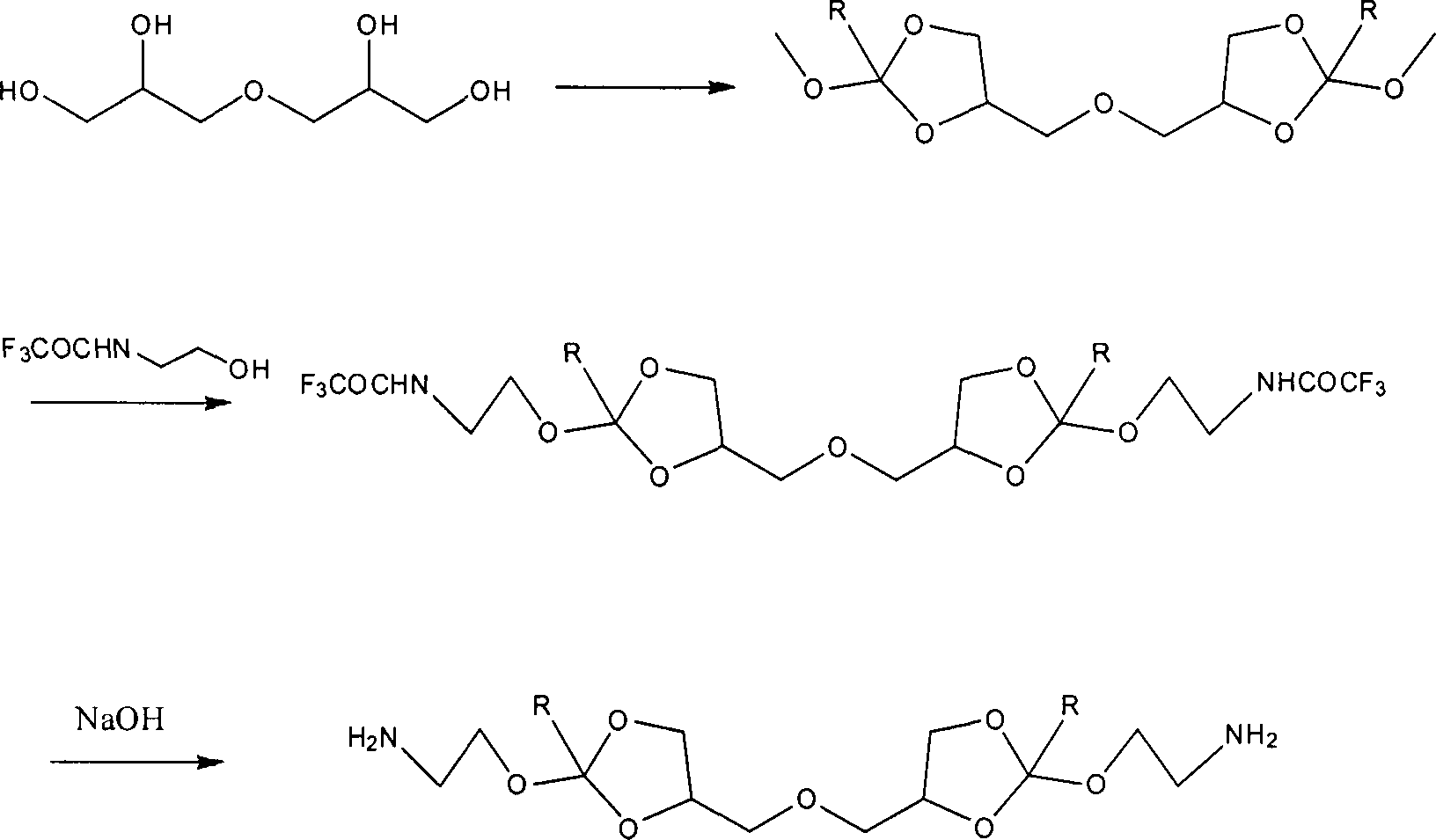
Synthesis method of cyclic orthoester monomer containing diamino
A synthetic method, diamino technology, applied in the fields of biomedicine and organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
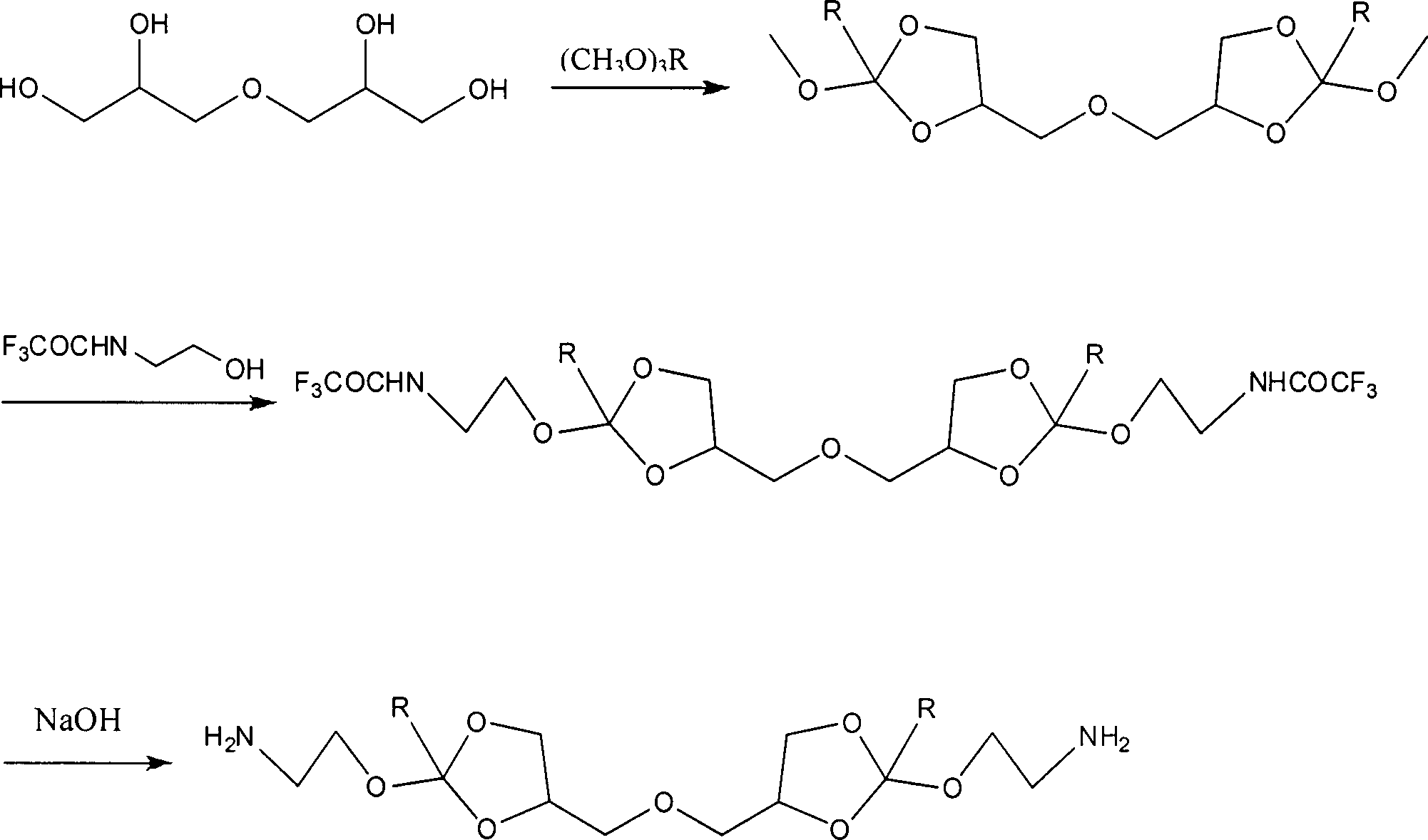
[0020] Preparation of 4,4'-dimethyleneoxy-bis-(2-methoxy-1,3-dioxolane)
[0021] Under the protection of nitrogen, add 10.0g (60.18mmol) diglycerin to a 250mL three-necked flask, add 100mL acetonitrile to dissolve, then add 51.08g (480mmmol), and finally add 260mg p-toluenesulfonic acid under stirring, and react overnight at room temperature. After the crude product was distilled off under reduced pressure to remove the organic solvent, it was dissolved by adding ethyl acetate, washed with 10% sodium carbonate solution, dried over magnesium sulfate, removed the organic solvent by distillation under reduced pressure, and vacuumized to obtain 14.93 g of a colorless oily product with a yield of 85.85%. . 1H NMR (400MHz, CDCl 3 ): 3.32-3.33 (s, 3H, OCH 3 ), 3.51-3.84 (m, 2H, OCH 2 ), 4.07-4.16 (m, 2H, OCH 2 ), 4.28-4.48 (m, 1H, OCHCH 2 ), 5.74-5.78 (t, 1H, OCHO)
[0022] 4,4'-Dimethyleneoxy-bis-[2,2,2-trifluoro-N-(2-methoxy-1,3-dioxolane-4-ethylene)acetamide]
[0023] Add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com