Method for preparing benzodiazepine compounds
A benzodiazepine and diazepine technology, which is applied in the field of preparation of benzodiazepine compounds, can solve the problems of high catalyst selectivity, high reaction temperature, large raw material damage, etc. Explosion risk, improved reaction yield, reduced hazard effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
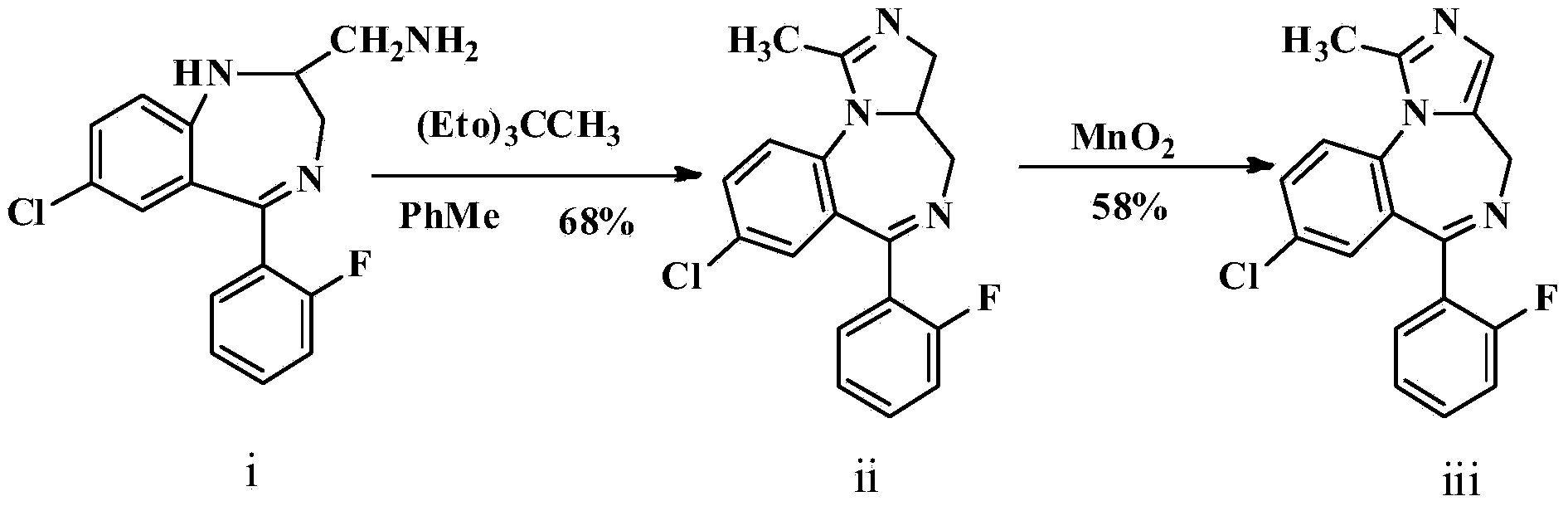
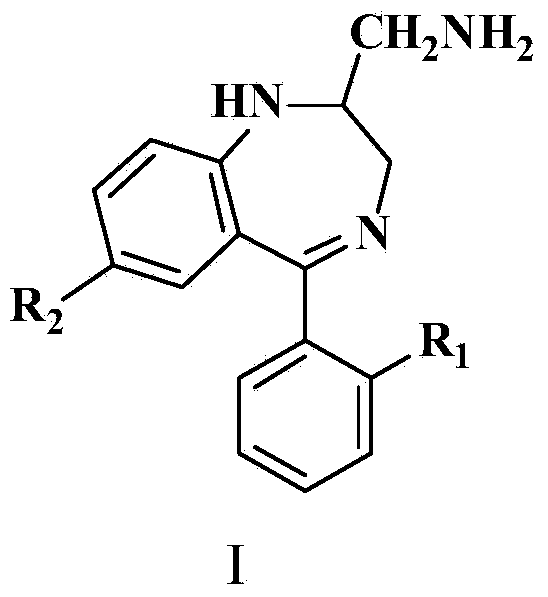
[0033]Add raw material I and triethyl orthoacetate in the ultrasonic reactor according to the amount shown in Table 1, set the ultrasonic power and ultrasonic time of the ultrasonic reactor, react at 20~30 ° C, adopt HPLC to track the reaction conversion rate, after the reaction is complete, from Release the reaction solution in the ultrasonic reactor, cool to below 20°C, stir for 3 hours to precipitate crystals, filter to obtain intermediate II, use hydrogen spectrum, carbon spectrum and other methods to detect intermediate II, confirm the structure of intermediate II, and calculate content and molar yield. The content is the amount of pure intermediate II in the obtained intermediate II, which is used to illustrate the amount of impurities. When the content is higher, the impurities are less. The molar feed ratio, ultrasonic power, ultrasonic time and yield of raw material I and triethyl orthoacetate are shown in Table 1.
[0034] Table 1 Preparation conditions and yield of...
Embodiment 19~45
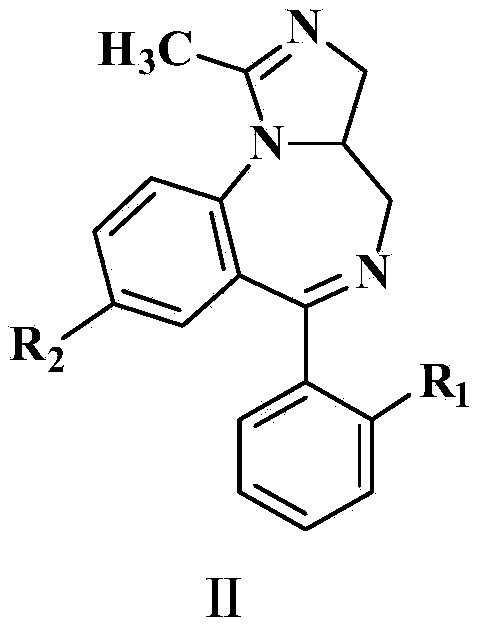
[0081] Add intermediate II and enzyme-containing bacterial cells obtained from different dehydrogenase-producing bacteria into the buffer solution, and perform biocatalysis according to the reaction time of 20h, reaction temperature of 34°C, stirring rate of 180r / min, and buffer solution pH value of 7.0 Dehydrogenation, to obtain the reaction solution containing product III, HPLC tracking conversion rate, HPLC detection conditions with embodiment 10 ~ 18, after the reaction, using toluene to extract the reaction solution, extraction 3 times, each extraction of toluene and reaction solution The volume ratio of the toluene phase is 1:1, the toluene phase is combined, and the toluene phase is concentrated to obtain the crude product III. The crude product is recrystallized to obtain the product III. The product III is detected by hydrogen spectrum, carbon spectrum and other methods to confirm the structure of the product III , and calculate the content and molar yield. Examples 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com