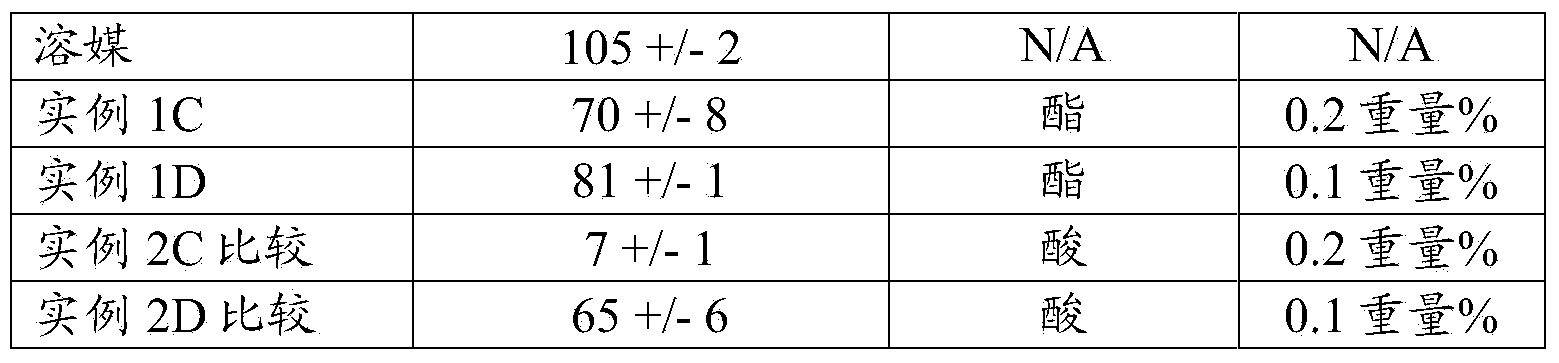Esters for treatment of ocular inflammatory conditions
An eye disease, ophthalmology technology, applied in the field of eye products, can solve problems such as bleeding time effects, increased cholesterol levels, disorders, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0128] The topical composition is formed by adding ethyl linolenate (alpha-linolenic acid ethyl ester (ALA-EE)), an esterified anti-inflammatory lipid mediator (AILM) having the following formula:
[0129] Added to a packaging solution / aqueous-based delivery system containing various surfactants / emulsifiers, wetting and chelating agents and one or more antioxidants, followed by mixing at high shear rates to provide Emulsion suitable for topical application. Table 4 summarizes the ingredients of the compositions.
[0130] Table 4:
[0131]
[0132] a=0.4% by weight boric acid, 0.2% by weight sodium borate, 0.5% by weight sodium chloride, 0.01% by weight diethylenetriaminepentaacetic acid (DTPA)
example 2
[0134] Compare
[0135] Topical compositions are formed by incorporating alpha-linolenic acid (ALA) into a packaging solution / aqueous based delivery system comprising various surfactants / emulsifiers, wetting and chelating agents and a or more antioxidants, followed by mixing at a high shear rate to provide an emulsion suitable for topical application. Table 5 summarizes the ingredients of the compositions.
[0136] table 5 :
[0137]
[0138] a=0.4% by weight boric acid, 0.2% by weight sodium borate, 0.5% by weight sodium chloride, 0.01% by weight diethylenetriaminepentaacetic acid (DTPA)
example 3
[0140] test
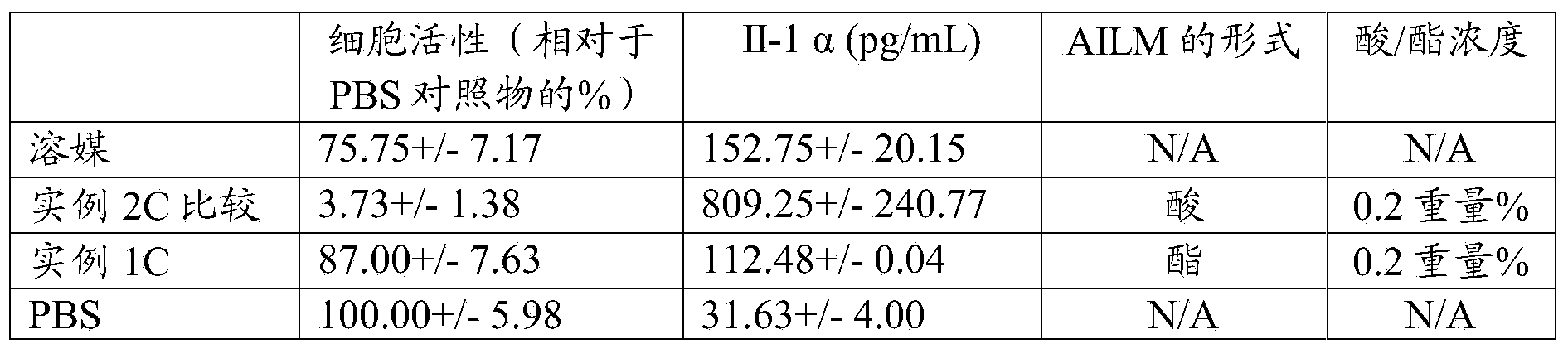
[0141] The compositions of Examples 1A and 1B and Comparative Example 2A were tested in vitro as follows. Fluorescein leakage (or increased transepithelial permeability) was evaluated using an in vitro model of transepithelial permeability (TEP test), in which the free acid and ester forms of α-linolenic acid were compared. The results are presented in Table 6, which shows that a high concentration (0.2%) of ALA ethyl ester performed similarly to the vehicle (packaging solution with Tween and Glucam) in effect on transepithelial permeability, while the same concentration of ALA fatty acid showed A significant increase in fluorescein leakage relative to controls was observed, consistent with clinical observations of discomfort during instillation. This suggests that, using concentrations up to 0.2%, omega-3 fatty acids in ester form will have improved instillation tolerability characteristics compared to the use of free acids.
[0142] Table 6
[0143] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com