Transformed and attenuated Listeria monocytogenes introducing human CD24 (cluster of differentiation 24) nucleotide sequence, and vaccine thereof
A nucleotide sequence, Listeria technology, applied in the field of attenuated Listeria, to achieve high safety and reliability, prevent drug resistance, and avoid the effect of chloramphenicol resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation method of attenuated Listeria vaccine
[0021] The strains in this example were provided by Professor Franckle of the University of Pennsylvania.
[0022] The medium / reagent that embodiment relates to:
[0023] Improved BHI (heart-brain perfusion fluid) medium: 100ml deionized water + 3.7g BHI dry powder + 20mg D-alanine;
[0024] BHIS (sucrose heart and brain perfusion solution): 100ml deionized water + 3.7g BHI dry powder + 17.115g sucrose + 20mg D-alanine;
[0025] SGWB (glycerol-sucrose buffer): 1000ml deionized water + 171.15g sucrose + 100ml glycerol + 0.2383g 4-hydroxyethylpiperazineethanesulfonic acid;
[0026] BHI medium: 100ml deionized water + 3.7g BHI dry powder;
[0027] BHI plate: 100ml+deionized water+3.7gBHI dry powder+100g agarose;
[0028] BHI chloramphenicol resistance medium: 100ml deionized water + 3.7g BHI dry powder + 1mg chloramphenicol;
[0029] BHI chloramphenicol resistance plate: 100ml deionized water + 3.7g BHI dr...
Embodiment 2
[0048] Example 2 The Second Homologous Recombination Experiment of Attenuated Listeria Secreting Human CD24 Protein
[0049] After the attenuated Listeria is transferred into the PKSV-CD24 shuttle plasmid by electroporation, it must be able to continuously and stably secrete CD24 protein to effectively activate the body's immune response in order to become a qualified vaccine.
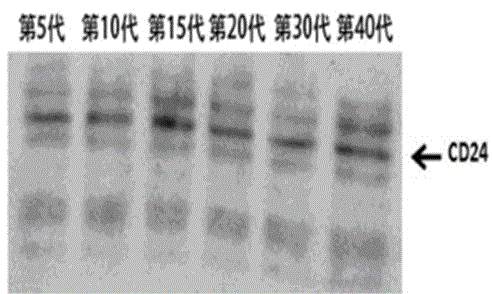
[0050] In the present invention, the second homologous recombination colony obtained in Example 1 is further cultured in BHI medium for 40 generations, and the 5th, 10th, 15th, 20th, 30th and 40th generation attenuated Listeria progenies are extracted respectively Bacteria culture medium, use TCA / acetone precipitation method to extract secreted protein in BHI medium, use western blot method to identify the protein in the expression of the protein for identification, the experimental results show that with the increase of the number of passages, the attenuated Listeria monocytogenes Can continuously and...
Embodiment 3
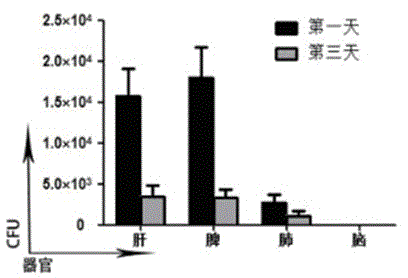
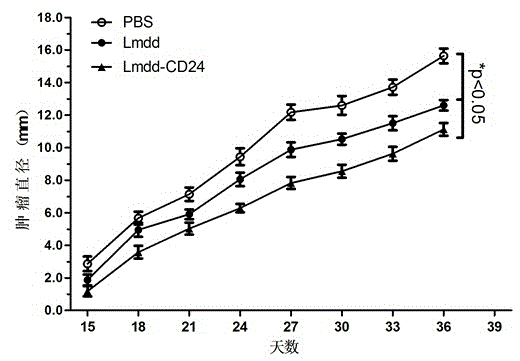
[0051] Example 3 Experiment of Infection Ability of Recombined Attenuated Listeria Live Vaccine to Cells and Tissues
[0052] Attenuated Listeria is a monocytogenes Gram-positive bacterium whose ability to infect host cells plays a key role in activating cellular and humoral immune responses.
[0053] The infectivity of the recombined attenuated Listeria vaccine in Example 1 was tested in vivo to observe whether exogenous gene recombination would affect the survival and reproduction of the vaccine strain. the way is:
[0054] Experimental group: the recombinant attenuated Listeria vaccine provided in Example 1;
[0055] Control group: attenuated Listeria vaccine provided by Professor Franckle of the University of Pennsylvania;
[0056] a) Select HepG2, Snu423, SMMC-7721, Huh-7 liver cancer cell lines and mouse macrophage cell line Raw264.7, and infect them in vitro with the vaccines of the experimental group and the control group. There was no significant difference in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com