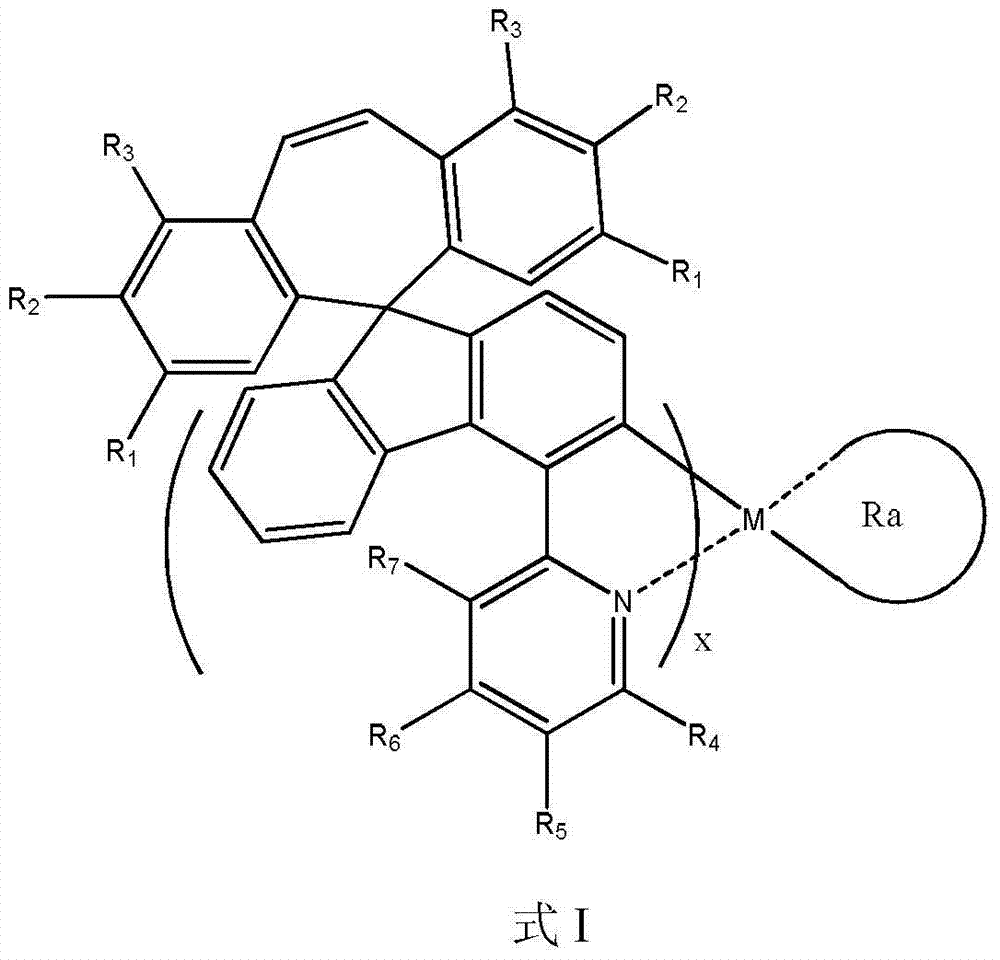
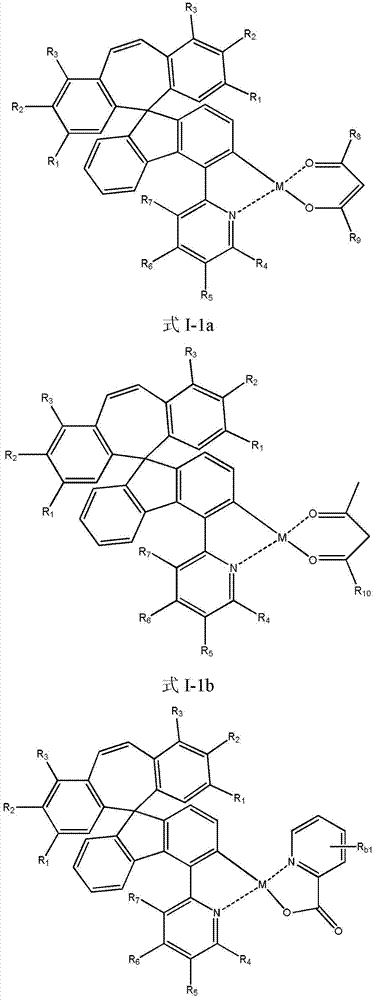
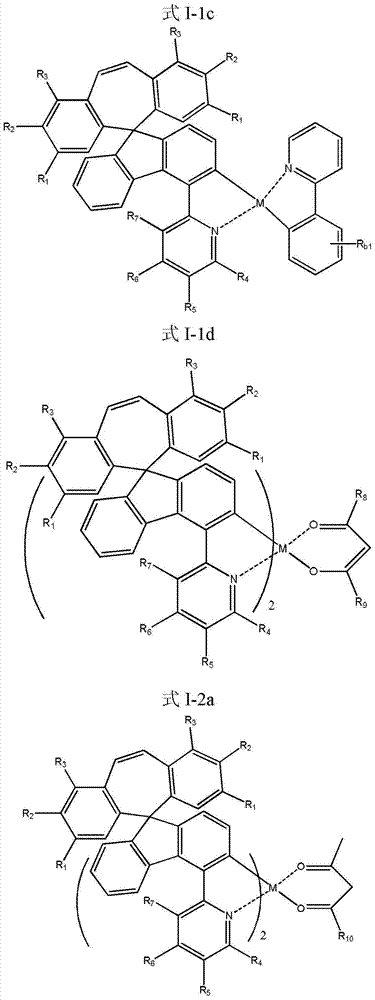
Organic phosphorescent OLED materials
A technology of luminescent materials and compounds, applied in luminescent materials, organic chemistry, electrical components, etc., can solve the problems of small luminous contribution and difficulty in improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Preparation of Example 1 Compound CJH-IRAC-001
[0086]
[0087] The first step: preparation of compound CJH-1
[0088]
[0089] Dissolve 3.12g of 2,2'-dibromobiphenyl in 500ml of anhydrous THF, cool down to -80°C with liquid nitrogen, and slowly add 4.4ml of 2.5M n-butyllithium-hexane solution dropwise under nitrogen protection , after stirring and reacting for 30 minutes, slowly add 2.06g of 5-dibenzocycloheptenone dissolved in THF solution dropwise, after stirring and reacting for 30 minutes, rise to room temperature and stir for 1 hour, add dropwise 150ml of saturated sodium bicarbonate The aqueous solution was extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain 4.4 g of a light yellow liquid, which was directly used in the next reaction without purification.
[0090] The second step: the preparation of compound CJH-2
[0091]
[0092] The light yellow liquid ...
Embodiment 2
[0117] Preparation of Example 2 Compound CJH-IRAC-005
[0118]
[0119]Referring to the fourth step of Example 1, the CJH-3 prepared in the third step of Example 1 and 2-bromo-6-picoline were used to prepare compound CJH-4, and the fifth to sixth steps of Example 1 were used to synthesize CJH-IRAC- 005, yellow solid.
[0120] Experimental data:
[0121] (1) Glass transition temperature (DSC): 237.65°C;
[0122] (2) UV maximum absorption wavelength (DCM): 305nm, 335nm, 358nm;
[0123] (3) Phosphorescence emission wavelength (DCM): 522nm.
Embodiment 3
[0124] Preparation of Example 3 Compound CJH-IRPY-001
[0125]
[0126] 2.13g of the compound CJH-5 prepared in Example 1, 707mg of 2-pyridinecarboxylic acid, 324mg of potassium carbonate-free and 50ml of 1,4-dioxane were heated and refluxed for 8 hours, then concentrated to dryness under reduced pressure. , the residue was separated and purified with a silica gel column to obtain 1.1 g of compound CJH-IRPY-001 as a yellow solid.
[0127] Experimental data:
[0128] (1) Glass transition temperature (DSC): 259.22°C;
[0129] (2) UV maximum absorption wavelength (DCM): 305nm, 335nm, 358nm;
[0130] (3) Phosphorescence emission wavelength (DCM): 525nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com