Catalytic cracking flue gas sulfur transfer agent adopting mixed crystal phases as well as preparation method and application thereof
A catalytic cracking and sulfur transfer agent technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of reducing the number of desulfurization active centers of sulfur transfer agents, secondary The function of silicon oxide to enhance strength declines, etc., to achieve the effect of enhancing desulfurization effect and service life, good desulfurization activity, and widening the composition range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
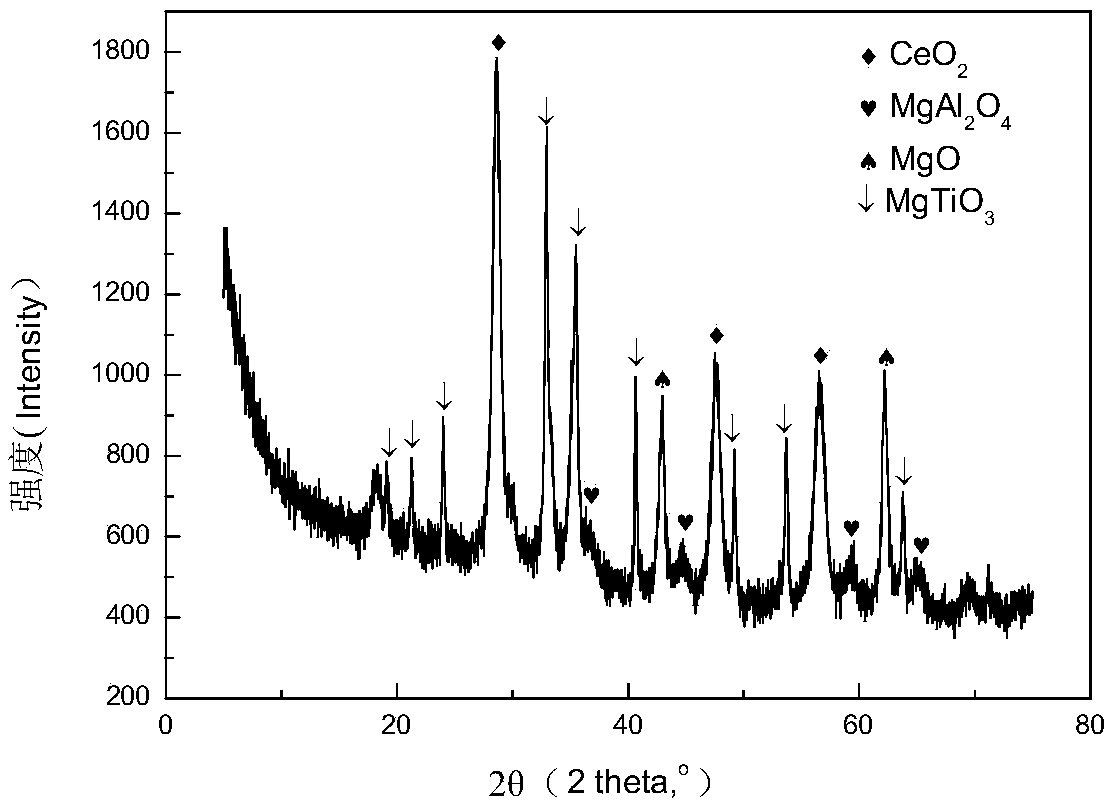
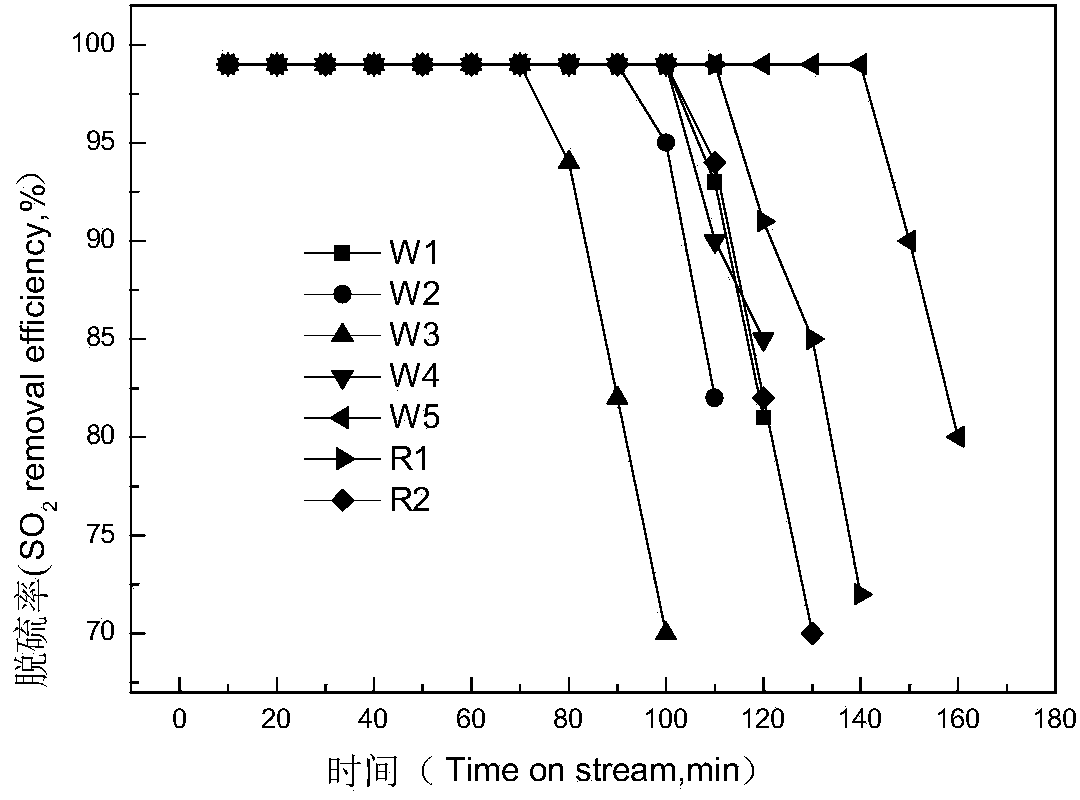
[0037] 43.77g MgSO 4 , 16.8g AlCl 3 , 30.16g Ti(SO 4 ) 2 9H 2 O, 15.15g Fe(NO 3 ) 3 9H 2 O, 9.06g Cu(NO 3 ) 2 ·3H 2 O dissolved in 200g H 2 O, prepared as solution A, and stirred at room temperature for 4h; dissolved 30g NaOH in 200g H 2 In O, configure solution B; add solution A dropwise to solution B heated in a 60°C water bath with vigorous stirring (to make the slurry rotate quickly, but not to prevent the slurry from flying out of the beaker), and continue stirring for 4 hours after the addition is completed , to obtain slurry C, keep the pH value of the slurry ≥ 10 during the whole mixing process, put slurry C into a reaction kettle with polytetrafluoroethylene lining, crystallize at 80°C for 18h, then cool, filter, and wash until neutral , dried at 120°C for 10h, and calcined at 700°C for 2h to obtain the product W1.
Embodiment 2
[0039] 22.8g MgSO 4 , 11.4g Mg(NO 3 ) 2 ·6H 2 O, 9.58g MgCO 3 , 17.47g Al 2 (SO 4 ) 3 , 40.24gTi(SO 4 ) 2 9H 2 O, 15.15g Fe(NO 3 ) 3 9H 2 O, 6.78g Ce(NO 3 ) 3 ·6H 2 O dissolved in 200g H 2 O, prepared solution A, and stirred at room temperature for 4h; dissolved 30g NaOH, 10g KOH in 200g H 2 In O, configure solution B; add solution A dropwise to solution B heated in a vigorously stirred 60°C water bath, continue stirring for 4 hours after the addition is complete, to obtain slurry C, and keep the pH value of the slurry ≥ 10 during the entire mixing process , Put the slurry C into a reaction kettle lined with polytetrafluoroethylene, crystallize at 90°C for 20h, then cool, filter with suction, wash until neutral, dry at 110°C for 10h, and roast at 700°C for 2h to obtain product W2.
Embodiment 3
[0041] 30.13g MgCl 2 ·6H 2 O, 38.54g C 4 h 6 o 4 Mg·6H 2 O, 16.8g AlCl 3 , 47.12g C 16 h 36 o 4 Ti, 6.77g Sr(NO 3 ) 2 , 3.67g Zn(NO 3 ) 2 ·6H 2 O was dissolved in 200g of absolute ethanol to prepare solution A, and stirred at room temperature for 4h; 25g NaOH, 15g KOH, 8g NaCO 3 Soluble in 200g H 2 In O, configure solution B; add solution A dropwise to solution B heated in a vigorously stirred 60°C water bath, continue stirring for 4 hours after the addition is complete, to obtain slurry C, and keep the pH value of the slurry ≥ 10 during the entire mixing process , put the slurry C into a reaction kettle lined with polytetrafluoroethylene, crystallize at 70°C for 17h, then cool, filter with suction, wash until neutral, dry at 110°C for 10h, and roast at 800°C for 2h to obtain product W3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com