Sulfonated aromatic diamine compound as well as preparation method and application thereof
A technology for sulfonating aromatic diamines and compounds, which is applied in the preparation of sulfonic acid, dehydration/demulsification by chemical methods, and organic chemistry, etc., can solve the problems of affecting the demulsification effect, etc. Affinity enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
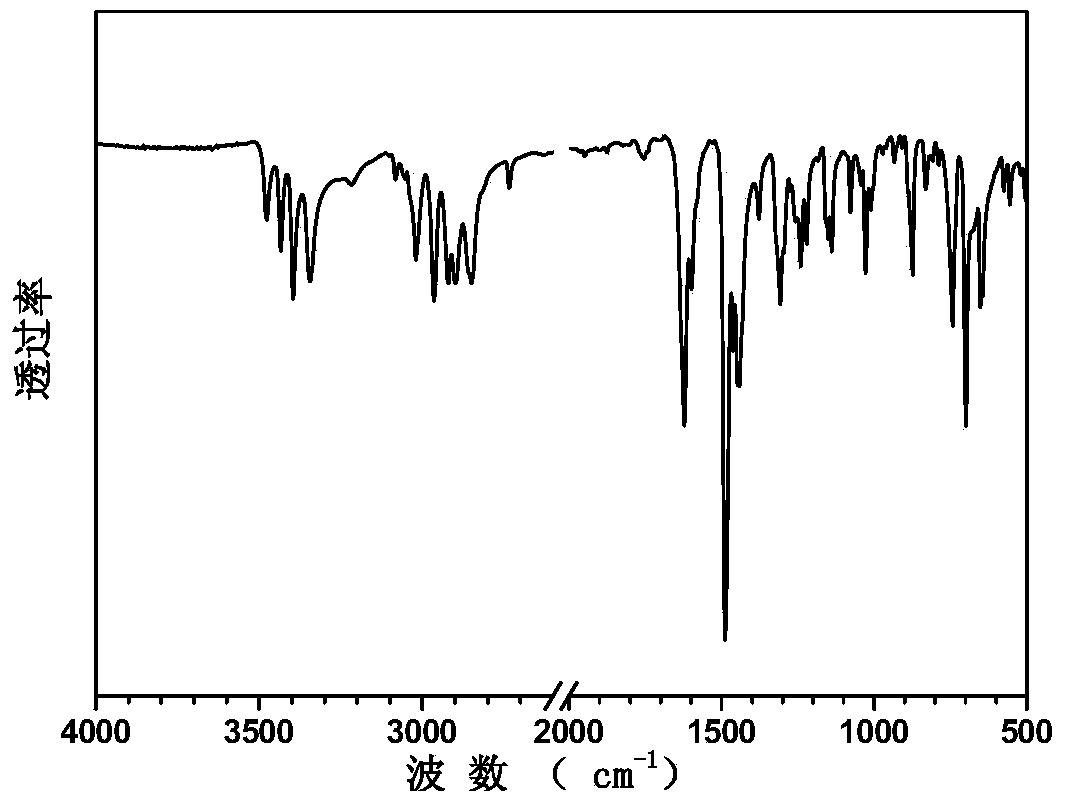
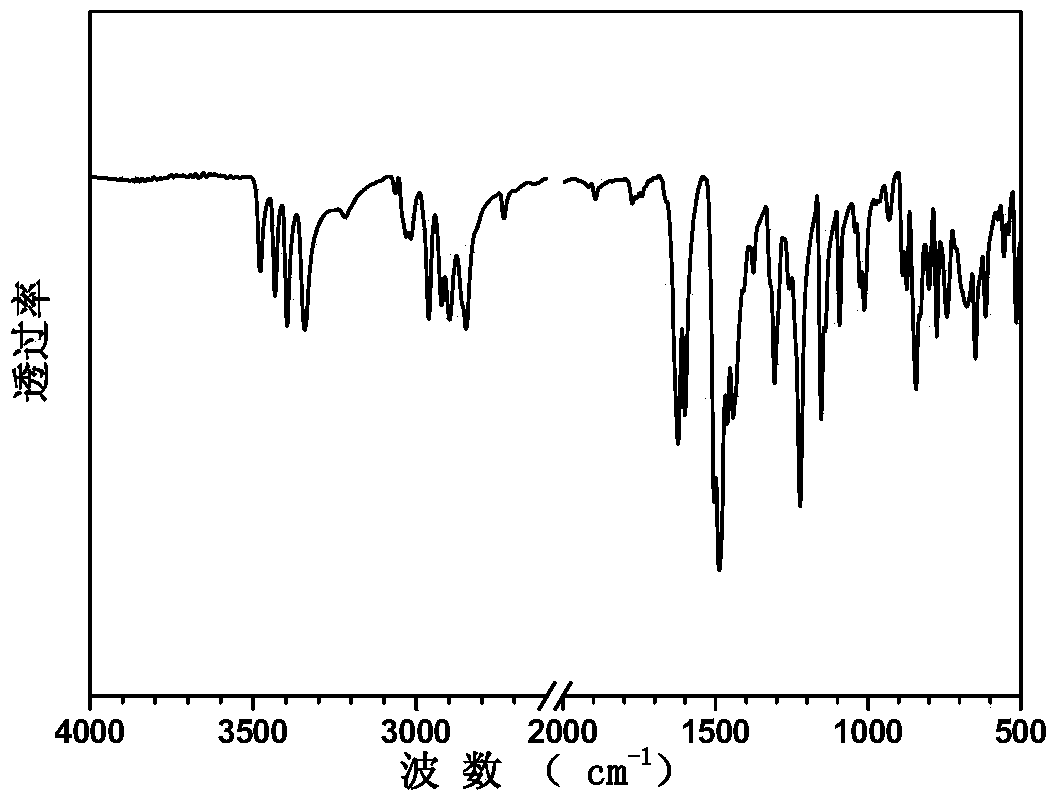
Image
Examples
Embodiment 1
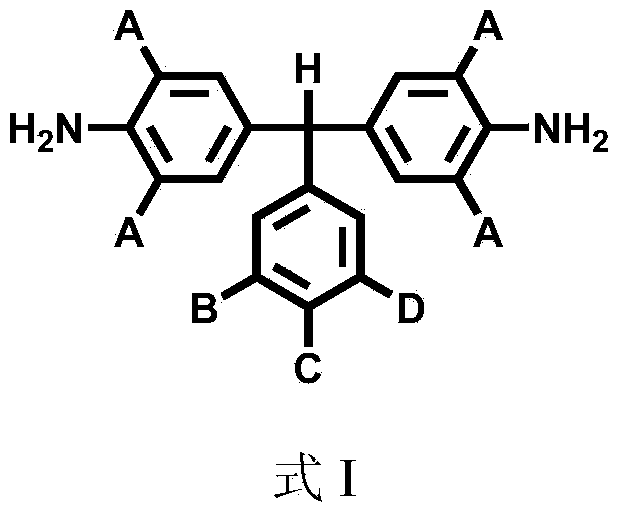
[0030] The synthesis of sulfonated aromatic diamine compound shown in embodiment 1, formula I
[0031] (1) Synthesis of aromatic diamines
[0032] Add 37.20g (0.40mol) of aniline and 70mL of distilled water to a three-necked flask equipped with mechanical stirring, a condenser, and a nitrogen port, and stir thoroughly under nitrogen protection. Slowly add 50.00mL concentrated hydrochloric acid (mass fraction 37%) dropwise, keep the system temperature at 40°C, then add 29.52g (0.22mol) 3,5-dimethylbenzaldehyde in batches. After the system was vigorously stirred under reflux for 12 hours, the temperature of the reaction system was lowered to 50° C., and 34.60 g of anhydrous potassium carbonate powder was added in batches under stirring until the system no longer produced bubbles. The solution was subjected to steam distillation, and a solid powder was obtained after suction filtration. Rinse the solid powder with hot water, recrystallize with ethanol, and dry to obtain 53.10 g...
Embodiment 2
[0037] Synthesis of sulfonated aromatic diamine compound shown in embodiment 2, formula I
[0038] (1) Synthesis of aromatic diamines
[0039] Add 48.47g (0.40mol) of 2,6-dimethylaniline and 60.00mL of distilled water to a three-necked flask equipped with mechanical stirring, a condenser, and a nitrogen port, and stir fully under nitrogen protection. 45.00 mL of concentrated hydrochloric acid (37% by mass) was slowly added dropwise, and the temperature of the system was maintained at 40°C, and then 22.28 g (0.21 mol) of benzaldehyde was added in batches. After the system was vigorously stirred under reflux for 8 hours, the temperature of the reaction system was lowered to 40° C., and 31.50 g of anhydrous potassium carbonate powder was added in batches under stirring until the system no longer produced bubbles. The solution was subjected to steam distillation, and a solid powder was obtained after suction filtration. Rinse the solid powder with hot water, recrystallize with e...
Embodiment 3
[0044] Synthesis of sulfonated aromatic diamine compound shown in embodiment 3, formula I
[0045] (1) Synthesis of aromatic diamines
[0046] Add 59.68g (0.40mol) of 2,6-diethylaniline and 120.00mL of distilled water to a three-necked flask equipped with mechanical stirring, a condenser, and a nitrogen port, and stir thoroughly under nitrogen protection. 61.50 mL of sulfuric acid solution (35% by mass) was slowly added dropwise, and the system temperature was maintained at 30°C, and then 28.56 g (0.21 mol) of 4-methoxybenzaldehyde was added in batches. After the system was vigorously stirred under reflux for 6 hours, the temperature of the reaction system was lowered to 50° C., and 36.90 g of sodium bicarbonate powder was added in batches under stirring until the system no longer produced bubbles. The solution was subjected to steam distillation, and a solid powder was obtained after suction filtration. Rinse the solid powder with hot water, recrystallize with ethanol, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dehydration rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com