Application and method of series of organic dyestuffs in visible-light photocatalyst Ru(bpy)3Cl2 fluorescence quenching
An organic dye and fluorescence quenching technology, applied in the field of fluorescence spectrum analysis, can solve the problems of few researches on fluorescence quenching, achieve good fluorescence quenching effect, simple and controllable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
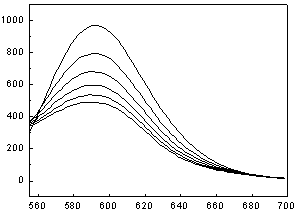
[0041] Dye Brilliant Blue E-FFN ( 1 ) to Ru(bpy) 3 Cl 2 Determination of Fluorescence Quenching in Aqueous Solutions
[0042] Accurately add 1 mL of the prepared Ru(bpy) with a pipette gun 3 Cl 2 Put the aqueous solution in a cuvette, cover the cuvette with a lid, and measure the emission spectrum F of the solution 0 ; Then add 1 μL of 0.001 mol / L dye Brilliant Blue E-FFN aqueous solution prepared in advance to the cuvette, mix well and measure the emission spectrum, observe the quenching situation, measure 1×10 -6 mol / L, 2×10 -6 mol / L, 3×10 -6 mol / L, 4×10 -6 mol / L, 5×10 -6 mol / L Fluorescence intensity F of the mixed solution at 5 different concentrations to obtain a set of quenched Ru(bpy) with corresponding concentrations 3 Cl 2 Solution fluorescence spectrum. Kg=49.3 / 10 10 mol ?1 · dm 3 ·s ?1 .
[0043] .
Embodiment 2
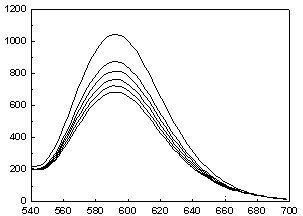
[0045] Cibazon P-6R( 2 ) to Ru(bpy) 3 Cl 2 Determination of Fluorescence Quenching in Aqueous Solutions
[0046] Accurately add 1 mL of the prepared Ru(bpy) with a pipette gun 3 Cl 2 Put the aqueous solution in a cuvette, cover the cuvette with a lid, and measure the emission spectrum F of the solution 0 ; Then add 1 μL of 0.001 mol / L aqueous solution of dye Cibazon P-6R prepared in advance to the cuvette, measure the emission spectrum after mixing evenly, observe the quenching situation, and measure 1×10 -6 mol / L, 2×10 -6 mol / L, 3×10 -6 mol / L, 4×10 -6 mol / L, 5×10 -6 mol / L Fluorescence intensity F of the mixed solution at 5 different concentrations to obtain a set of quenched Ru(bpy) with corresponding concentrations 3 Cl 2 Solution fluorescence spectrum. Kg=25.7 / 10 10 mol ?1 · dm 3 ·s ?1 .
[0047] .
Embodiment 3
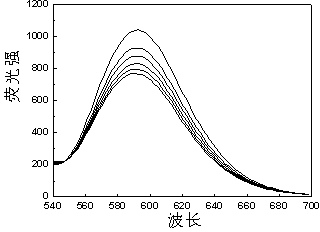
[0049]Reactive Brilliant Blue FN-G( 3 ) to Ru(bpy) 3 Cl 2 Determination of Fluorescence Quenching in Aqueous Solutions
[0050] Accurately add 1 mL of the prepared Ru(bpy) with a pipette gun 3 Cl 2 Put the aqueous solution in a cuvette, cover the cuvette with a lid, and measure the emission spectrum F of the solution 0 ; Then add 1 μL of 0.001 mol / L aqueous solution of dye reactive brilliant blue FN-G prepared in advance to the cuvette, measure the emission spectrum after mixing evenly, observe the quenching situation, measure 1×10 -6 mol / L, 2×10 -6 mol / L, 3×10 -6 mol / L, 4×10 -6 mol / L, 5×10 -6 mol / L Fluorescence intensity F of the mixed solution at 5 different concentrations to obtain a set of quenched Ru(bpy) with corresponding concentrations 3 Cl 2 Solution fluorescence spectrum. Kg=17.9 / 10 10 mol ?1 · dm 3 ·s ?1 .
[0051] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com