Preparation method for 5'-methoxylaudanosine
A technology of methoxylaudansu and phosphorus oxychloride, which is applied in the direction of organic chemistry, can solve the problems of high recovery energy consumption, high energy consumption, and expensive methyl iodide, so as to increase production rate, reduce three wastes, and reduce energy consumption. consumption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
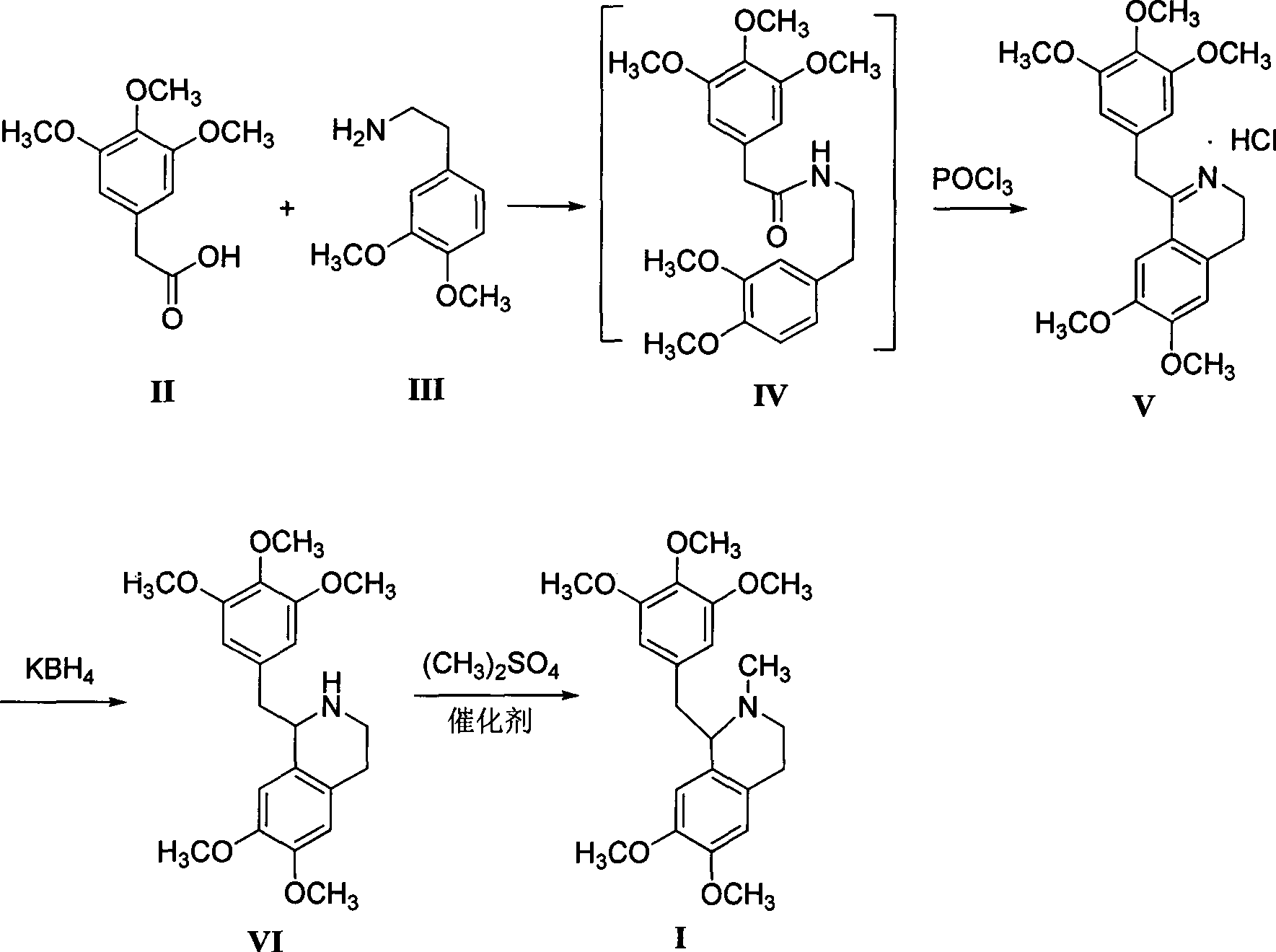
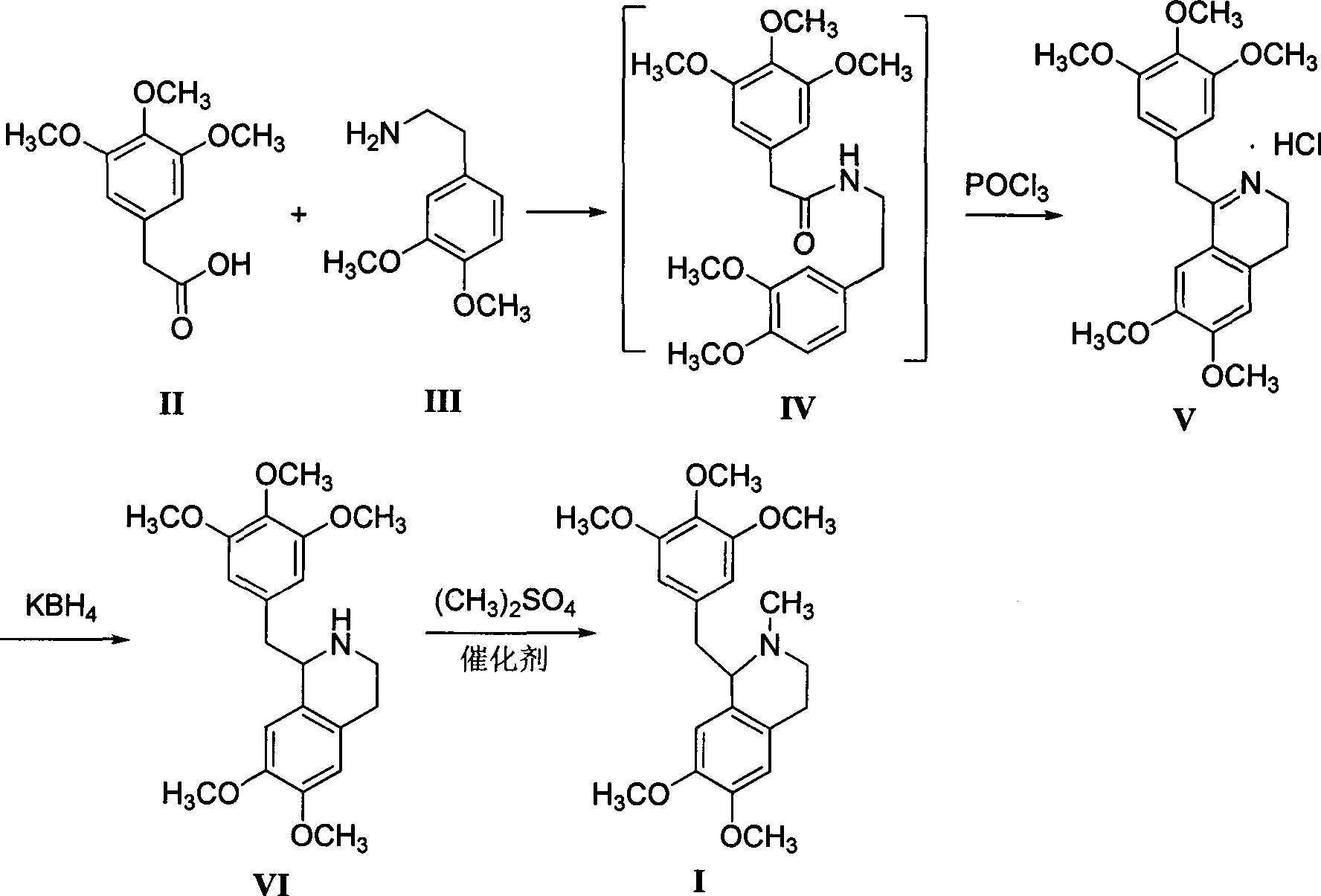
[0021] Preparation of 3,4-dihydro-6,7-2methoxy-1-[2-(3,4,5-trimethoxyphenyl)ethyl]isoquinoline hydrochloride
[0022] Add 600mL xylene, 22.6g (0.1mol) of 3,4,5-trimethoxyphenylacetic acid and 18.1g (0.1mol) of 3,4-dimethoxyphenethylamine successively in a 1L four-necked flask, and reflux Divide water for 5 hours, cool to 80-85°C, add 16.9g (0.11mol) of phosphorus oxychloride dropwise, keep warm for about 3-4 hours after dropping, after wave layer chromatography detection shows that the raw materials disappear, cool to 0-5°C, Filter, wash the filter cake with 50 mL of ethyl acetate, and dry to obtain off-white solid V (39.0 g, 96.2%), mp 109.7~111.3 °C 1,2,3,4-tetrahydro-6,7-dimethoxy - Preparation of 1-[2-(3,4,5-trimethoxyphenyl)ethyl]isoquinoline
[0023] Add 600mL of methanol and 2.3g (0.1mol) of sodium metal to a 1L four-neck flask in turn, stir until the sodium disappears, then cool to room temperature, add 40.6g (0.1mol) of compound V, stir for 10min to obtain a white tu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com