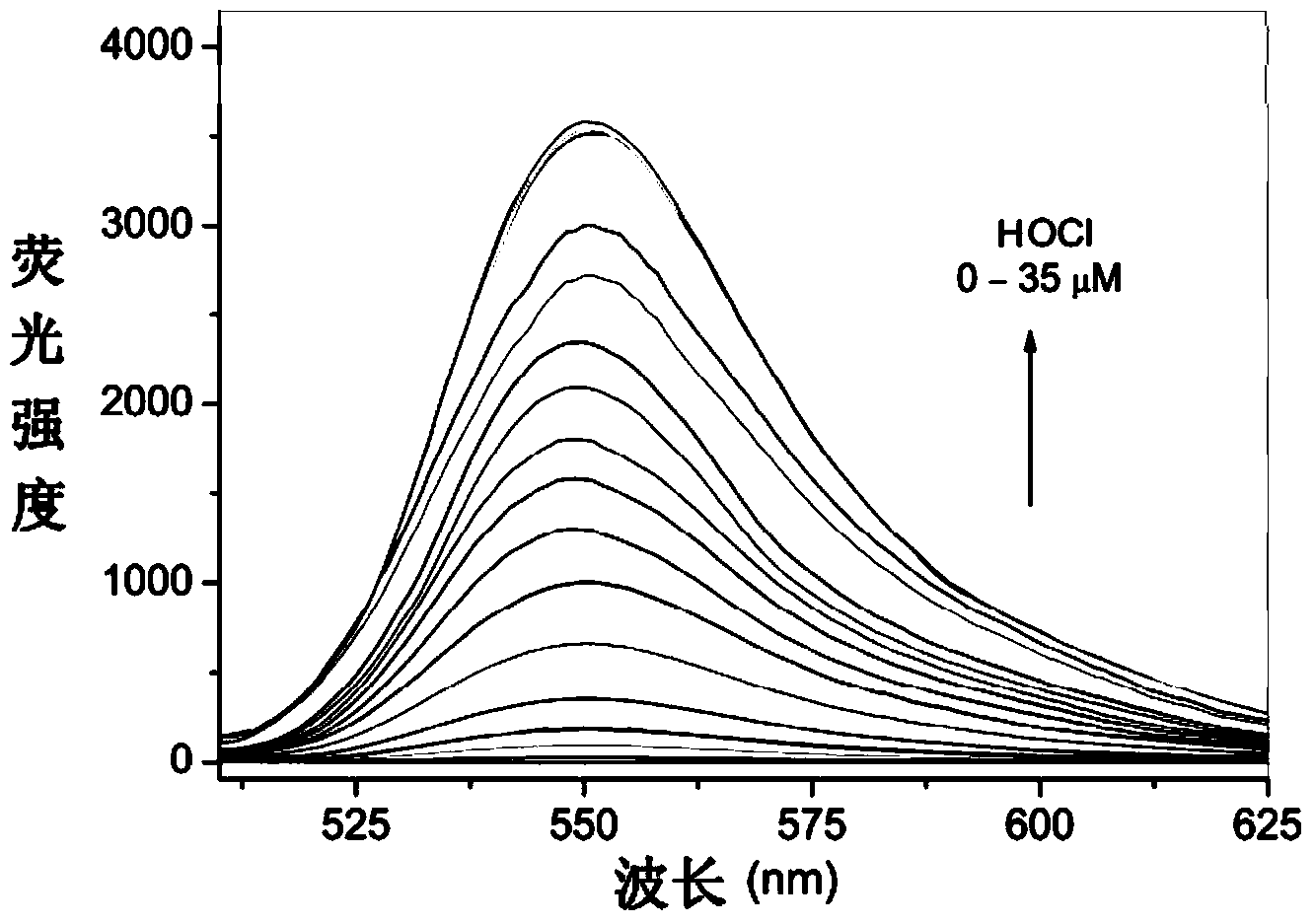
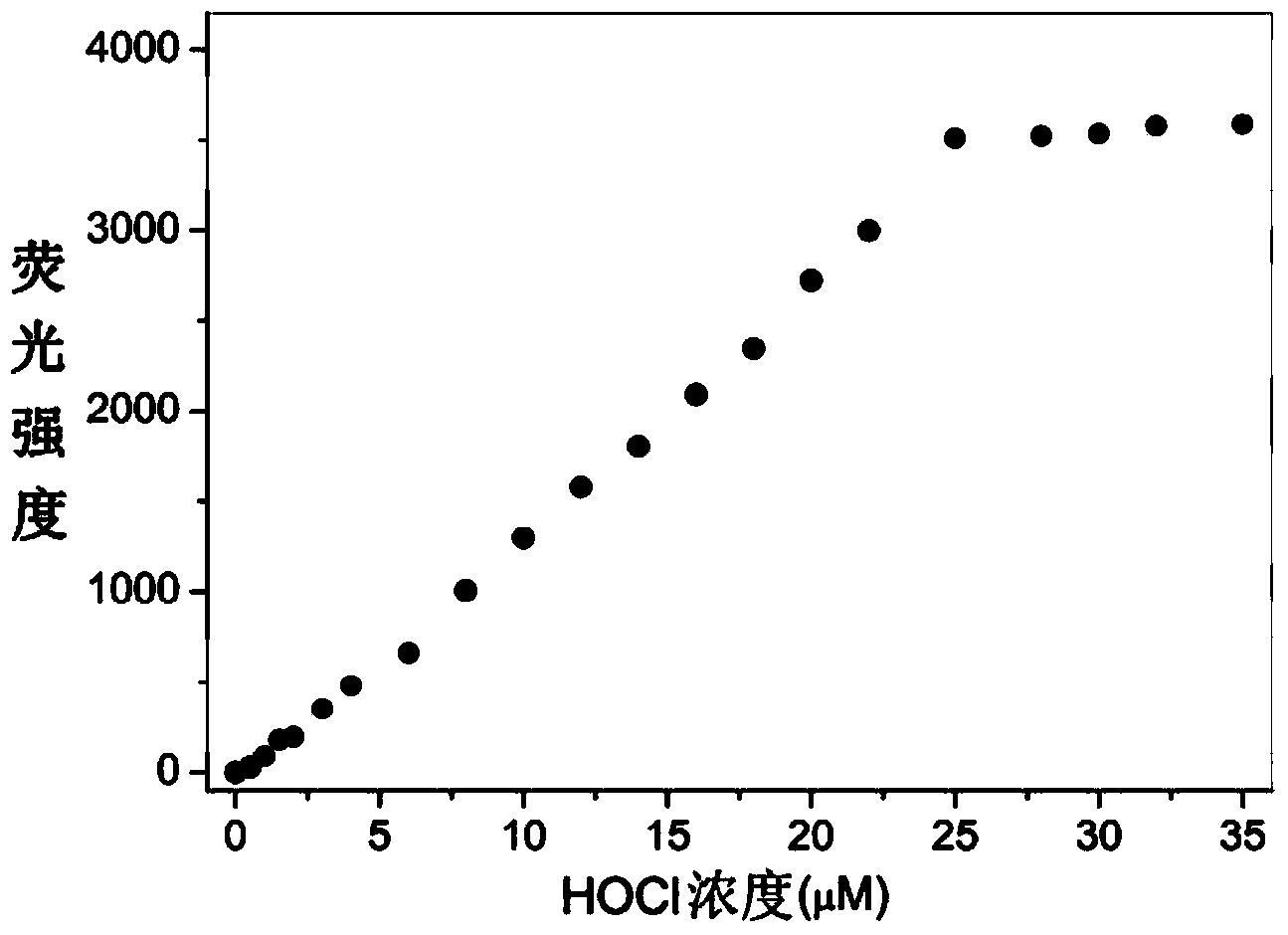
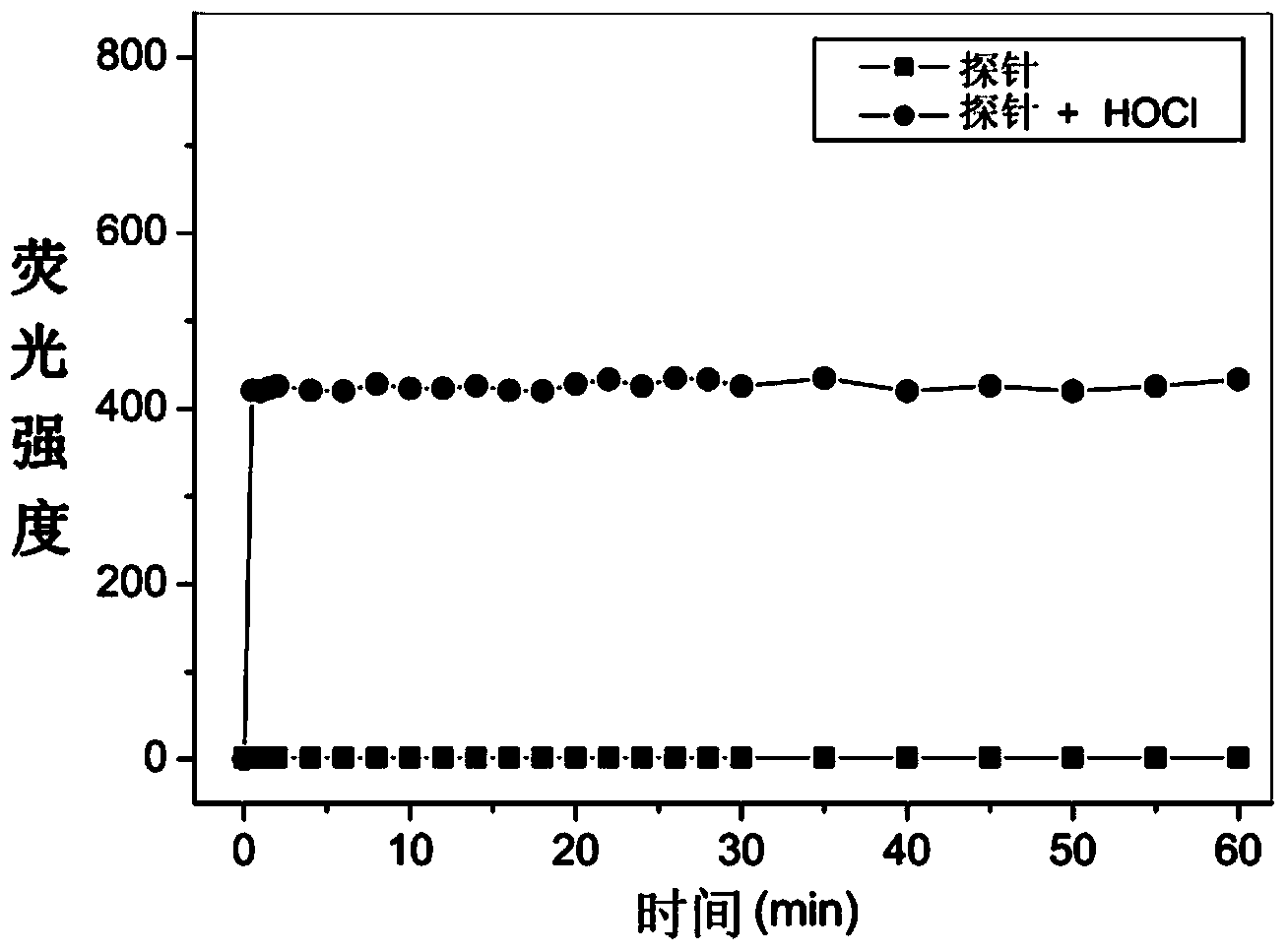
Rhodamine 6G hydrazide derivative, preparation method of derivative and application of derivative, and method for carrying out fluorescence analysis on hypochlorous acid by using derivative as fluorescence probe
A technology for hydrazide derivatives and fluorescence analysis, which is applied in the fields of fluorescence/phosphorescence, biological testing, material excitation analysis, etc., can solve problems such as cumbersome procedures, restrictions on practical applications, and complex technologies, and achieve broad application prospects and stable fluorescence detection signals , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of rhodamine 6G hydrazide derivative of the present invention comprises the following steps:
[0033] 1) Weighing a certain amount of rhodamine 6G, ethanol and hydrazine monohydrate with a mass concentration of 80%, and dissolving rhodamine 6G in ethanol, then adding hydrazine monohydrate dropwise, and then refluxing and filtering to obtain After precipitation, washing and drying, rhodamine 6G hydrazide was obtained as a pink solid, wherein the ratio of rhodamine 6G, ethanol and hydrazine monohydrate was 1.0g: 20mL: 3mL;
[0034] 2) Take a certain amount of 1-naphthoyl chloride and acetonitrile, then dissolve 1-naphthoyl chloride in acetonitrile to obtain an acetonitrile solution of 1-naphthoyl chloride, wherein 1-naphthalene in the acetonitrile solution of 1-naphthoyl chloride The ratio of formyl chloride to acetonitrile is 100 μL: 15 mL;
[0035] 3) Weigh a certain amount of rhodamine 6G hydrazide and acetonitrile, then dissolve rhodamine 6G hy...
Embodiment 2
[0041] The preparation method of rhodamine 6G hydrazide derivative of the present invention comprises the following steps:
[0042] 1) Weighing a certain amount of rhodamine 6G, ethanol and hydrazine monohydrate with a mass concentration of 80%, and dissolving rhodamine 6G in ethanol, then adding hydrazine monohydrate dropwise, and then refluxing and filtering to obtain After precipitation, washing and drying, rhodamine 6G hydrazide was obtained as a pink solid, wherein the ratio of rhodamine 6G, ethanol and hydrazine monohydrate was 1.0g: 30mL: 2mL;
[0043] 2) Take a certain amount of 1-naphthoyl chloride and acetonitrile, then dissolve 1-naphthoyl chloride in acetonitrile to obtain an acetonitrile solution of 1-naphthoyl chloride, wherein 1-naphthalene in the acetonitrile solution of 1-naphthoyl chloride The ratio of formyl chloride to acetonitrile is 90 μL: 13 mL;
[0044] 3) Weigh a certain amount of rhodamine 6G hydrazide and acetonitrile, then dissolve rhodamine 6G hydra...
Embodiment 3
[0050] The preparation method of rhodamine 6G hydrazide derivative of the present invention comprises the following steps:
[0051] 1) Weighing a certain amount of rhodamine 6G, ethanol and hydrazine monohydrate with a mass concentration of 80%, and dissolving rhodamine 6G in ethanol, then adding hydrazine monohydrate dropwise, and then refluxing and filtering to obtain After precipitation, washing and drying, rhodamine 6G hydrazide was obtained as a pink solid, wherein the ratio of rhodamine 6G, ethanol and hydrazine monohydrate was 1.0g: 25mL: 4mL;
[0052] 2) Take a certain amount of 1-naphthoyl chloride and acetonitrile, then dissolve 1-naphthoyl chloride in acetonitrile to obtain an acetonitrile solution of 1-naphthoyl chloride, wherein 1-naphthalene in the acetonitrile solution of 1-naphthoyl chloride The ratio of formyl chloride to acetonitrile is 115 μL: 11 mL;
[0053] 3) Weigh a certain amount of rhodamine 6G hydrazide and acetonitrile, then dissolve rhodamine 6G hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com