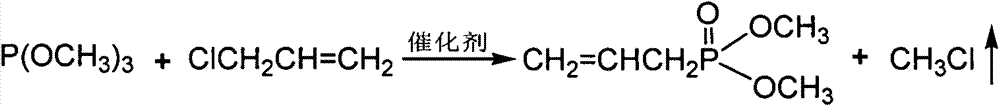
Method for preparing allyl phosphonic acid dimethyl ester
A technology of dimethyl allyl phosphonate and trimethyl phosphite, which is applied in the field of preparation of dimethyl allyl phosphonate, can solve the problems of no suitable preparation method, limited application and popularization, and poor reaction selectivity. Achieve good application development prospects, increase reaction yield, and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
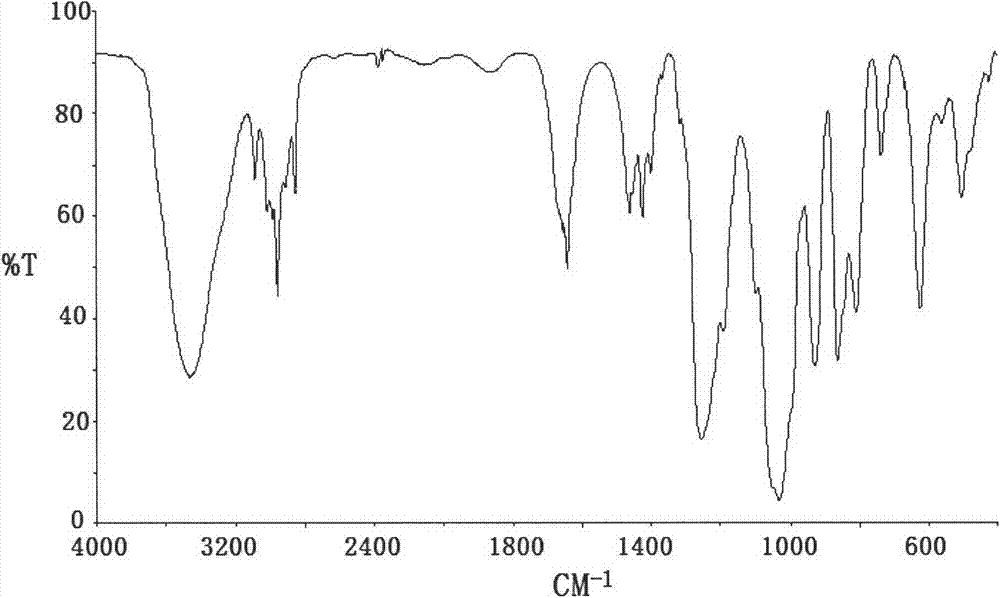
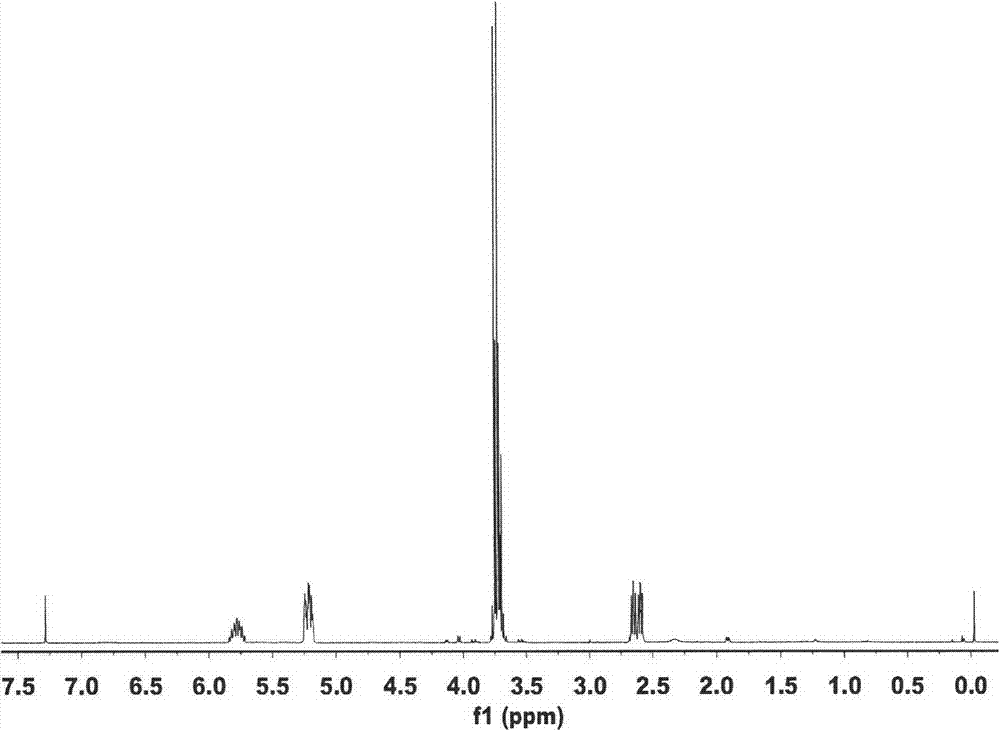
[0025] Example 1 Set up a 250mL four-necked flask equipped with a stirrer, thermometer, high-efficiency reflux condenser and methyl chloride collector, replace the air in the device with nitrogen, add 150ml of chlorobenzene, 12.4g (0.1mol) With trimethyl phosphate, 0.075gKI and 0.01g hydroquinone, the temperature was raised to 110℃, and within 4h, 9.2g (0.12mol) chloropropene was uniformly injected by a subsurface micro pump. After the injection is completed, the reaction is kept at 110°C for 4h, the excess chloropropene is recovered by distillation, and then the solvent is removed by distillation under reduced pressure (recycling), and then the fraction at 88-92°C under the pressure of 3950Pa is collected to obtain the product dimethyl allylphosphonic acid ester. The yield is 75.6%, boiling point: 182°C, refractive index: n D 25 =1.4387, density (25℃): 1.049g / cm 3 .
Embodiment 2
[0026] Example 2 Set up a 250mL four-necked flask equipped with a stirrer, thermometer, high-efficiency reflux condenser and methyl chloride collector, replace the air in the device with nitrogen, add 100ml DMF, 12.4g (0.1mol) phosphorous acid With trimethyl ester, 0.075g Na I and 0.01g hydroquinone, the temperature was raised to 110°C, and within 5 hours, 10.72g (0.14mol) of chloropropene was uniformly injected by a micro pump below the liquid surface. After the injection is completed, the temperature is raised to 130°C and the reaction is kept for 4 hours. The excess chloropropene is distilled to recover, and then the solvent is removed under reduced pressure (recovery), and then the fraction at 88-92°C under the pressure of 3950Pa is collected to obtain the product allylphosphonic acid two Methyl ester. The yield is 84.7%, boiling point: 182°C, refractive index: n D 25 =1.4387, density (25℃): 1.049g / cm 3 .
Embodiment 3
[0027] Example 3 Set up a 250mL four-necked flask equipped with a stirrer, thermometer, high-efficiency reflux condenser and methyl chloride collector, replace the air in the device with nitrogen, add 150ml diethylene glycol dimethyl ether, 12.4g (0.1mol) trimethyl phosphite, 0.1g CuCl and 0.01g hydroquinone, raise the temperature to 110°C, and inject 12.25g (0.16mol) chloropropene evenly in a subsurface micro pump within 4h. After the injection, the temperature is raised to 160°C and the reaction is kept for 5 hours. The excess chloropropene is distilled to recover, and then the solvent is removed under reduced pressure (recycling), and then the fraction at 88-92°C under the pressure of 3950Pa is collected to obtain the product allylphosphonic acid two Methyl ester. The yield is 83.2%, boiling point: 182°C, refractive index: n D 25 =1.4387, density (25℃): 1.049g / cm 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com