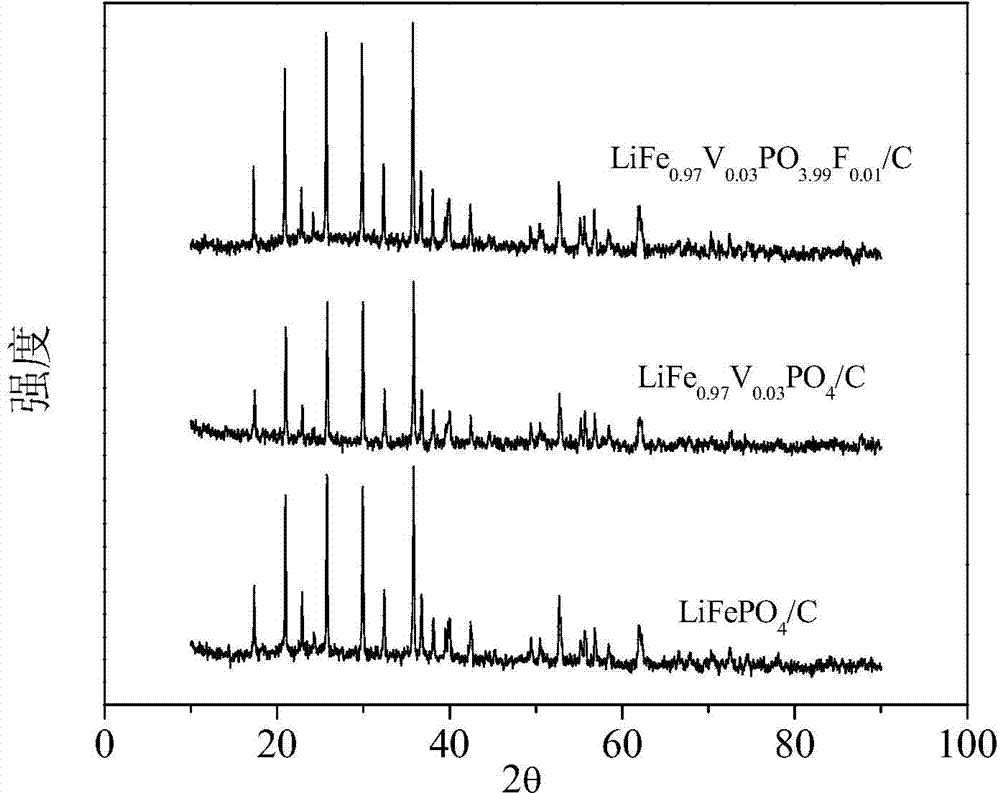
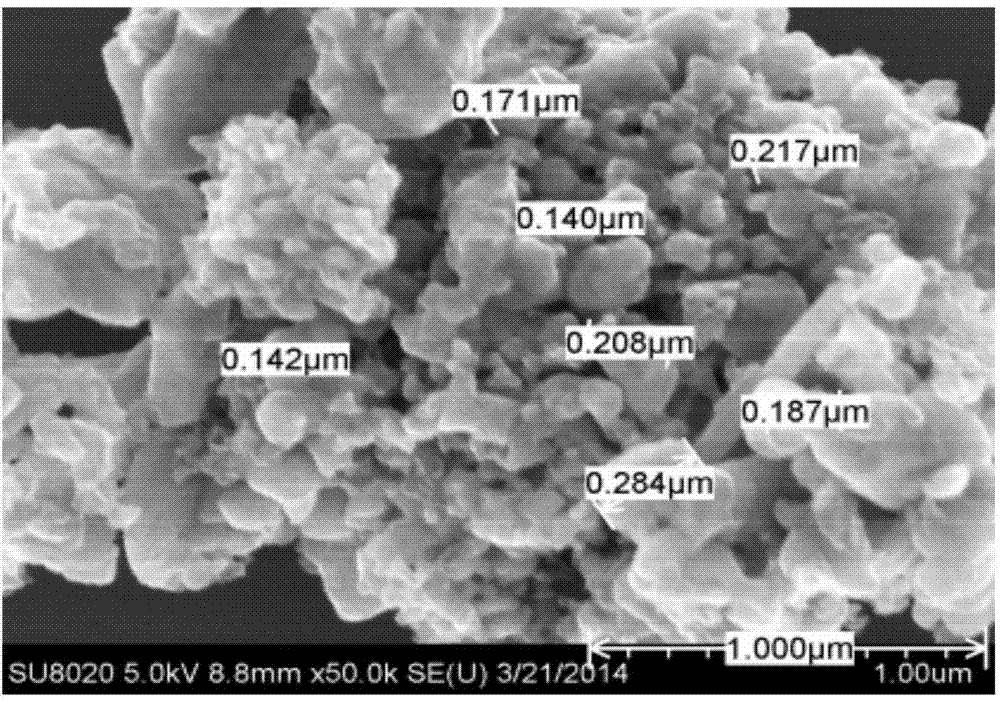
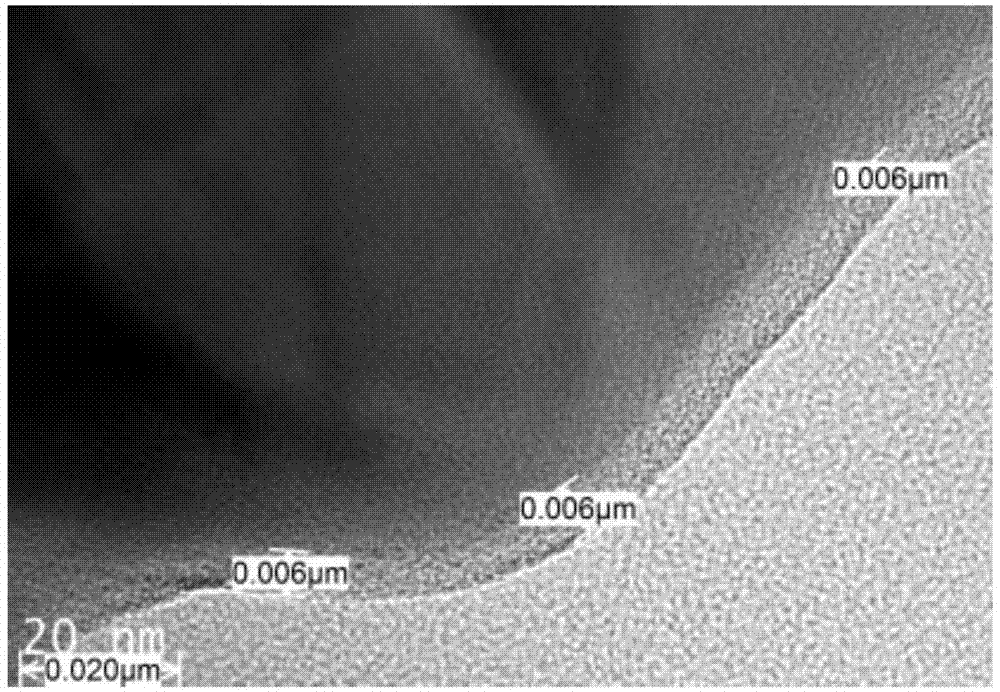
Anion and cation doped carbon-coated lithium iron phosphate cathode material and preparation method thereof
A technology for carbon-coated lithium iron phosphate and cathode materials, which is applied in chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve the problems of poor stability and low specific capacity of lithium iron phosphate cathode materials, and improve electrochemical performance. , the effect of reducing particle radius and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This embodiment prepares lithium iron phosphate cathode material according to the following steps:
[0032] Press Li + : Fe 2+ :PO 4 3- =1.05:1:1 ion molar ratio weighed 0.2440gLiOH, 1.6705gFe(CH 3 COO) 2 , 1.4319g (NH 4 ) 3 PO 4 Put the solid powder into a ball mill jar, add 2mL of absolute ethanol as a mixed medium, ball mill at a speed of 300r / min for 12 hours, put it into a blast drying oven, dry it at 55°C for 30 minutes, and then grind it by hand for half an hour, then put it into a quartz tube (Both ends are plugged with copper balls and a gap is left so that nitrogen can drive out the air in the quartz tube), and placed in a tube furnace under a nitrogen atmosphere (the flow rate of nitrogen is 2mL / min), at a rate of 1°C / 3min. The temperature was raised to 350°C, and after 6 hours of heat preservation, it was naturally lowered to room temperature to obtain a precursor; the obtained precursor was put into a ball mill tank, 6 mL of absolute ethanol and 0.1...
Embodiment 2
[0037] Press Li + : Fe 2+ :PO 4 3- =1.05:1:1 ion molar ratio weighed 0.3764gLi respectively 2 CO 3 , 1.1047gFeC 2 o 4 , 1.7278gNH 4 h 2 PO 4Put the solid powder into a ball mill jar, add 2mL of acetone as a mixed medium, ball mill at a speed of 300r / min for 12 hours, put it into a blast drying oven, dry it at 55°C for 30 minutes, grind it by hand for half an hour, and put it into a quartz tube (two ends Plug it with a copper ball and leave a gap so that nitrogen can drive out the air in the quartz tube), place it in a tube furnace under a nitrogen atmosphere (the flow rate of nitrogen is 2mL / min), and heat up at a rate of 1°C / 3min to 350°C, keep warm for 6 hours and then lower to normal temperature to obtain the precursor; put the obtained precursor into a ball mill tank, add 6mL acetone and 0.12g asphalt, ball mill at a speed of 300r / min for 12 hours, then put it into a blast drying oven, After drying at 55°C for 30 minutes, grind it by hand for half an hour, put it...
Embodiment 3
[0043] Press Li + : Fe 2+ : PO 4 3- =1.05:1:1 ion molar ratio weighed 0.3764gLi respectively 2 CO 3 , 1.6705gFe(CH 3 COO) 2 , 1.2683g (NH 4 ) 2 HPO 4 Put the solid powder into a ball mill jar, add 2mL cyclohexane as a mixed medium, ball mill at a speed of 300r / min for 12 hours, put it into a blast drying oven, dry it at 55°C for 30 minutes, grind it by hand for half an hour, and put it into a quartz tube , both ends were plugged with copper balls, placed in a tube furnace under a nitrogen atmosphere, the temperature was raised to 350°C at a rate of 1°C / 3min, and the temperature was lowered to room temperature after 6 hours of heat preservation to obtain a precursor; the obtained precursor was put into a ball mill tank , add 6mL cyclohexane and 0.135g asphalt, ball mill at 300r / min for 12 hours, put it into a blast drying oven, dry it at 55°C for 30min, grind it by hand for half an hour, put it into a quartz tube (both ends are plugged with copper balls) and leave a g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com