Preparation method of multicrystal LiFePO4 powder having olivine structure
A technology of olivine structure and H2PO4, which is applied in the direction of structural parts, chemical instruments and methods, rare earth metal compounds, etc., can solve the problems that are not conducive to the electrochemical performance of materials, the purity of synthetic materials is not high, and the particle size is large, so as to improve electronic performance. The effect of electrical conductivity, fine particles, and low firing temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention is a polycrystalline LiFePO with olivine structure 4 powder preparation method, the LiFePO 4 The powder is based on Fe(Ac) 2 , FeSO 4 、7H 2 O, Ba(Ac) 2 And organic acid is raw material, adopts sol-gel method to synthesize, and its specific implementation steps are:
[0022] (1)Fe(Ac) 2 Preparation: FeSO 4 ·7H 2 O is used as a source of ferrous iron, and an equimolar amount of FeSO is weighed 4 ·7H 2 O and Ba(Ac) 2 Put it in two beakers, add deionized water to dissolve it and make a solution of 0.001 ~ 1Mol / L. In FeSO 4 ·7H 2 Add 0.05-1% reduced iron powder to the O solution. Then the two solutions were mixed, stirred evenly and then centrifuged. The separated clear solution was collected and evaporated under nitrogen to give Fe(Ac) 2 powder.
[0023] (2) Weigh Fe(Ac) in molar ratio 2 :LiA:(NH 4 )H 2 PO 4 : Organic acid M=1:1:1:(1~16) (M=tartaric acid, gluconic acid or citric acid); Fe(Ac) 2 Make a solution of 0.001~0.1Mol / L, then add M...
Embodiment 1
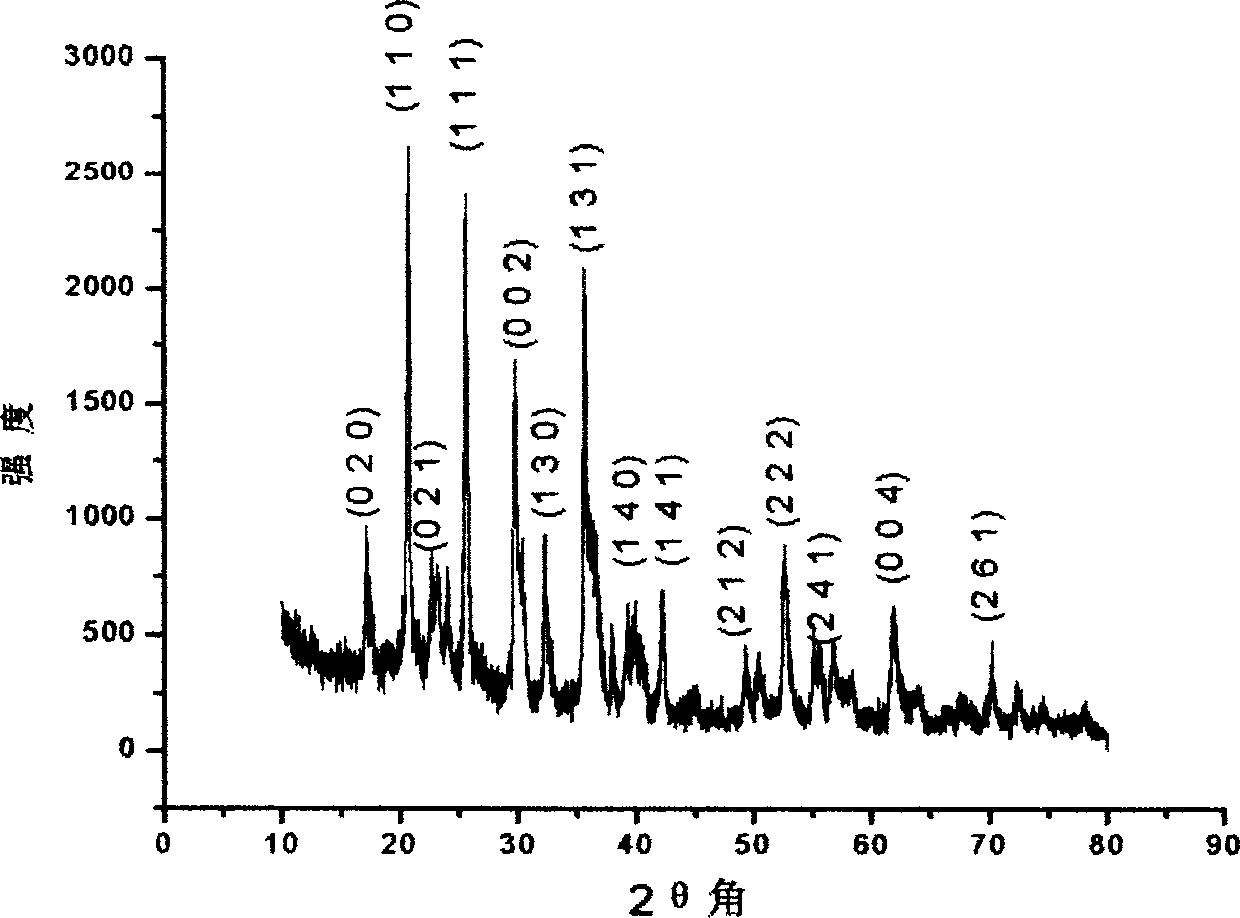
[0029] Embodiment 1: Take by weighing 0.05 mole of Fe(Ac) 2 Put the powder into an 800mL Erlenmeyer flask, add deionized water to make a 0.1Mol / L solution, and add 0.4M organic acid to Fe(Ac) 2 in solution. Stir and place in an oven and pass nitrogen gas at a rate of 0.16 L / Min, keep the temperature at 80°C, and obtain a yellow-green clear solution after 60 Min. To this clear solution was added 0.05 molar LiAc and stirred to dissolve. Then add 0.05 mole (NH 4 )H 2 PO 4 After stirring, the system becomes a light yellow-green solution after dissolution. Put the Erlenmeyer flask into an insulation jacket, keep the temperature at 100°C and also pass through it with nitrogen. After 2.0 hours, a dark green sol was obtained, and the heating was continued to obtain a gel block. Put the gel in a graphite crucible, heat it at 600°C for 5 hours to obtain a black porous loose block, and grind it finely to obtain LiFePO4 Powder material. Figure (1) is the XRD spectrum of the materi...
Embodiment 2
[0030] Embodiment 2: Take by weighing 0.03 moles of Fe(Ac) 2 Put the powder into an 800mL Erlenmeyer flask, add deionized water to dissolve it to form a 0.05Mol / L solution, and add 0.12M organic acid. Stir and place in an oven and pass nitrogen gas at a rate of 0.16 L / Min, keep the temperature at 80°C, and obtain a green clear solution after 40 Min. To this clear solution was added 0.03 molar LiAc and stirred to dissolve. Then add 0.03 mol (NH 4 )H 2 PO 4 After stirring, the system becomes a light yellow-green solution after dissolution. Put the Erlenmeyer flask into an insulation jacket, keep the temperature at 80°C and also pass through it with nitrogen. After 3.5 hours, a dark green sol was obtained, and the heating was continued to obtain a gel block. Put the gel in a graphite crucible, heat it at 500°C for 5 hours to obtain a black porous loose block, and grind it finely to obtain LiFePO 4 Powder material. Figure (3) is the SEM image of the material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com