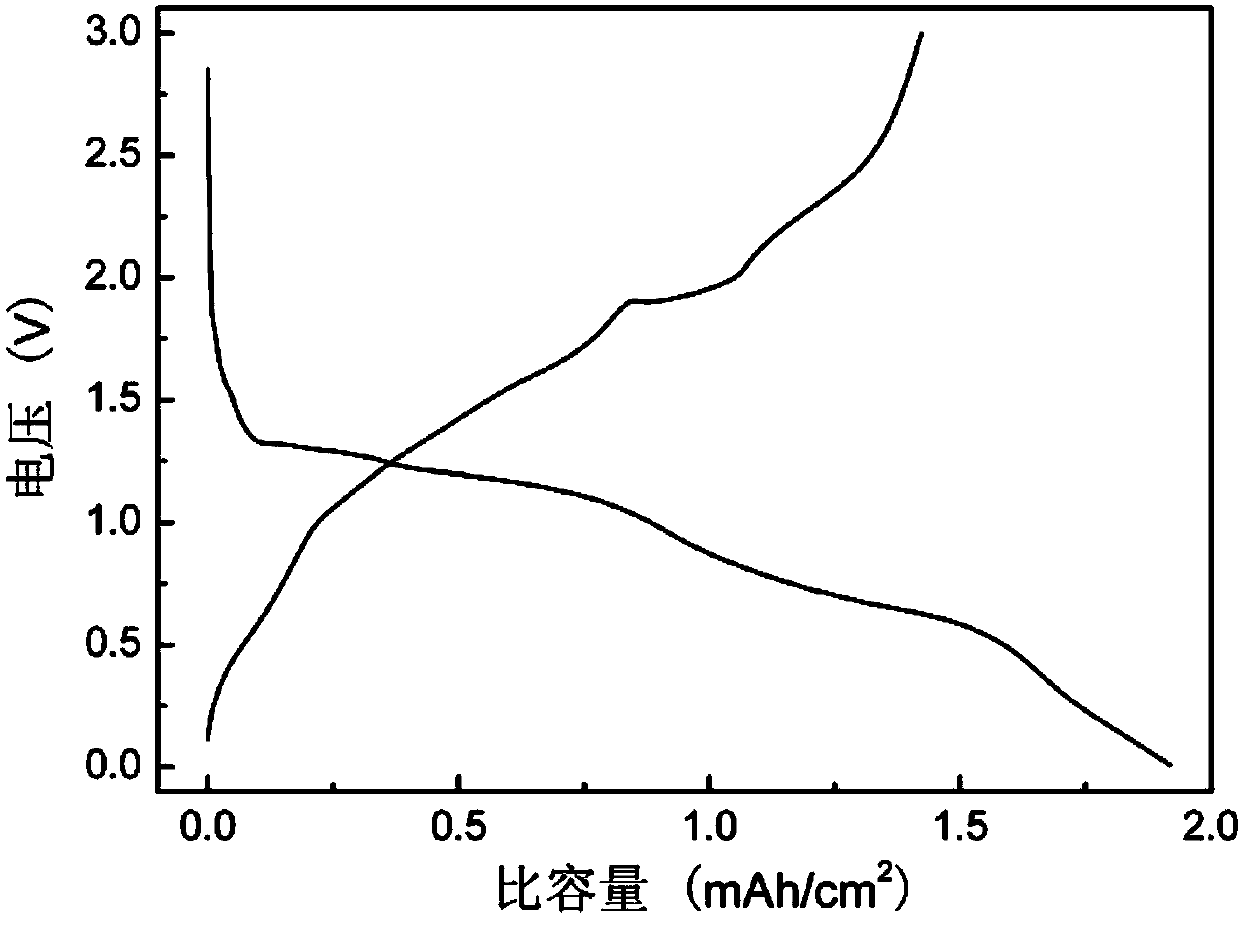
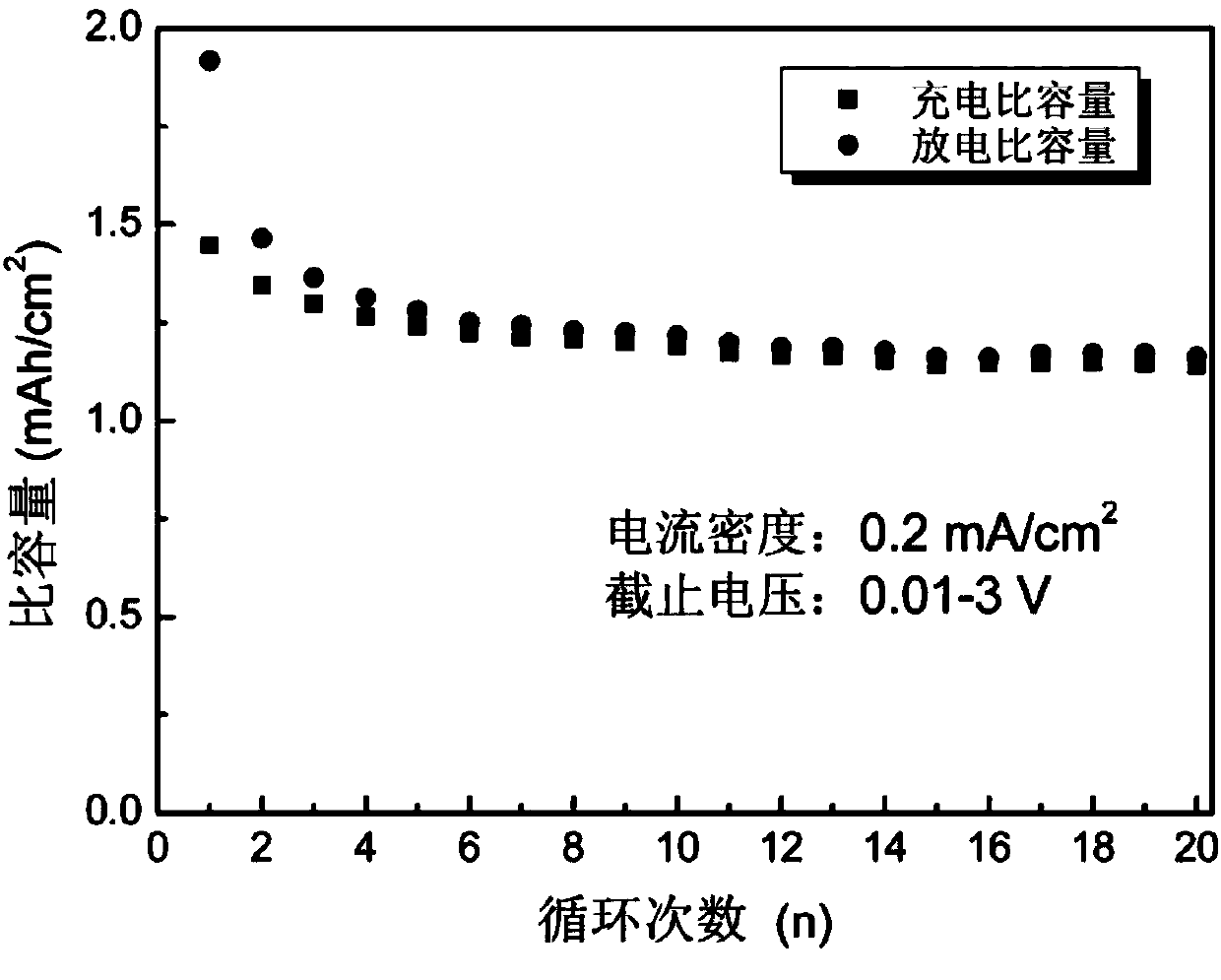
Preparation method of nano Ni3S2 material with lamellar structure
A lamellar structure and nanotechnology, applied in the field of nano-Ni3S2 materials and their preparation, can solve the problems of unfavorable large-scale production, toxic raw materials, and complicated process, and achieve excellent electrochemical performance, low cost, and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 0.19 g NiCl 2 ·6H 2 O, 0.10 g of urea was dissolved in 70 ml of deionized water, stirred evenly to form a light green clear solution. The mixed solution was continuously stirred for 1 h, and then the obtained clear solution was poured into a 100 ml hydrothermal kettle with a polytetrafluoroethylene liner, and a piece of Ni mesh was placed in the polytetrafluoroethylene liner. o C for 18 h. The precursor obtained by the reaction was washed with deionized water and ethanol, respectively, and vacuum-conditioned at 50 o C to dry for 2 h. Weigh 0.14 g Na 2 S·9H 2 O was dissolved in 70 ml of deionized water, stirred evenly to form a clear solution, and then the obtained clear solution was poured into a 100 ml hydrothermal kettle with a polytetrafluoroethylene liner, and the precursor obtained above was put into it. o C for 7 h. The product obtained by the reaction was washed with deionized water and ethanol respectively, and was vacuum-conditioned at 50 o After...
Embodiment 2
[0032] Weigh 0.48 g Ni(NO 3 ) 2 ·6H 2 O, 0.19 g of ammonia water was dissolved in 70 ml of absolute ethanol, and stirred evenly to form a light green clear solution. The mixed solution was continuously stirred for 1 h, and then the obtained clear solution was poured into a 100 ml hydrothermal kettle with a polytetrafluoroethylene liner, and a piece of Ni mesh was placed in the polytetrafluoroethylene liner, at 180 o C for 10 h. The precursor obtained by the reaction was washed with deionized water and ethanol, respectively, and vacuum-conditioned at 50 o C to dry for 2 h. Weigh 0.2 g SnS 2 Dissolve in 70 ml of absolute ethanol, stir evenly to form a clear solution, then pour the resulting clear solution into a 100 ml hydrothermal kettle with a polytetrafluoroethylene liner, and put the precursor obtained above, at 180 o C for 18 h. The product obtained by the reaction was washed with deionized water and ethanol respectively, and was vacuum-conditioned at 50 o After d...
Embodiment 3
[0035] Weigh 0.6 g Ni(CH 3 COO) 2 4H 2 O, 0.9 g of urea was dissolved in 70 ml of ethanol and alcohol mixture (1:1, V / V), and stirred evenly to form a light green clear solution. The mixed solution was continuously stirred for 1 h, and then the obtained clear solution was poured into a 100 ml hydrothermal kettle with a polytetrafluoroethylene liner, and a piece of Ni mesh was placed in the polytetrafluoroethylene liner. o C for 12 h. The precursor obtained by the reaction was washed with deionized water and ethanol, respectively, and vacuum-conditioned at 50 o C to dry for 2 h. Weigh 0.7 g CoS and dissolve it in 70 ml ethanol and alcohol mixture (1:1, V / V), stir well to form a clear solution, then pour the obtained clear solution into a 100 ml Teflon-lined In the water heating still, and put into the above-mentioned obtained precursor, at 160 o C for 7 h. The product obtained by the reaction was washed with deionized water and ethanol respectively, and was vacuum-condit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com