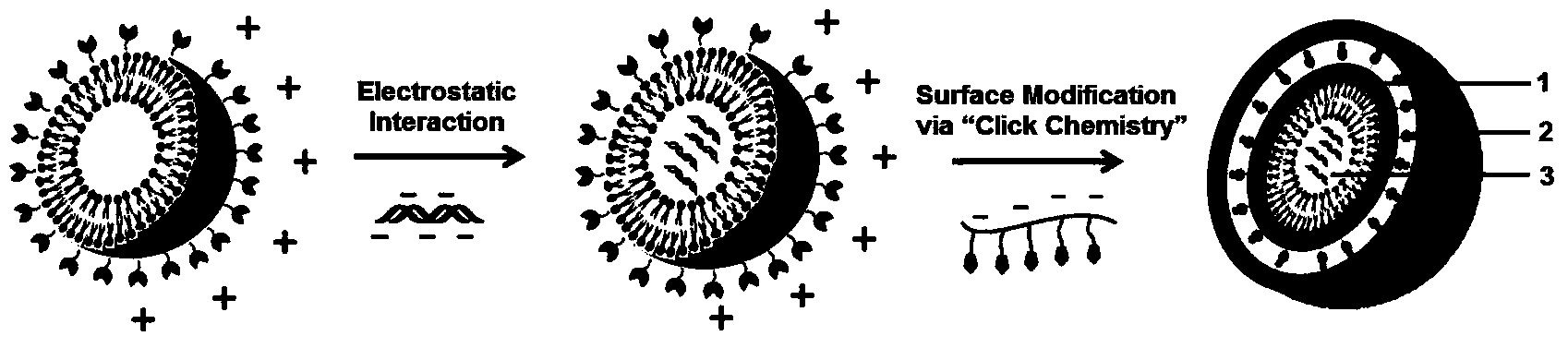
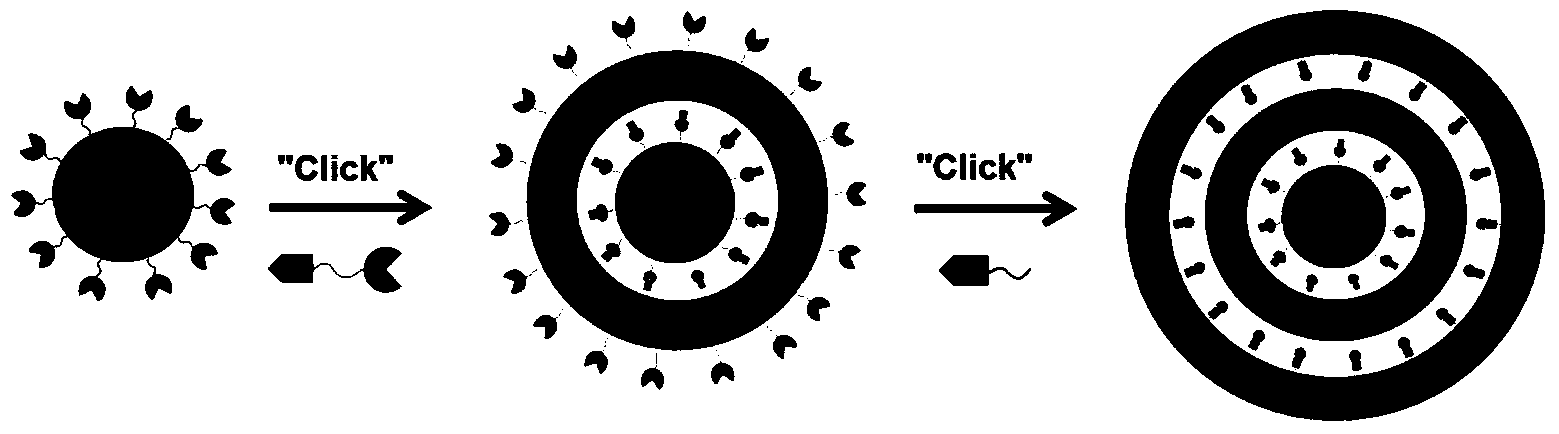
Gene-therapy drug delivery system based on cooperative assembling
A drug and alkynylation technology, applied in gene therapy, drug combination, pharmaceutical formulation, etc., can solve the problems of poor stability of the coating layer, difficulty in stable and effective assembly, poor specificity and recognition ability, and improve stability and targeted effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of azidated dipalmitoylphosphatidylethanolamine
[0067] Chloroethylamine hydrochloride (5 g, 43.1 mmol) and sodium azide (8.4 g, 129 mmol) were dissolved in 30 mL of water, and reacted at 80° C. for 15 h. After the reaction, solid potassium hydroxide was added to adjust the pH of the reaction solution to 12-14, extracted with ether, and the organic layer was concentrated to obtain a light yellow oil (2.8 g, 75.6%). The light yellow oil (2.8g, 32mmol) and hexahydrophthalic anhydride (4.16g, 27mmol) were dissolved in 50mL of chloroform, reacted at 25°C for 5h, and subjected to dichloromethane / methanol column chromatography to obtain a white powdery solid (hexaazide hydrobenzoic acid, 5.9 g, 90.9%). Azidohexahydrobenzoic acid (0.38g, 1.57mmol) and N-hydroxysuccinimide (0.36g, 3.14mmol) were dissolved in 20mL of chloroform, and 20mL of 1-ethyl-(3-bis Methylaminopropyl) carbodiimide hydrochloride (0.6g, 3.14mmol), activated for 1h, adding dipalmitoylphosphatid...
Embodiment 2
[0070] Preparation of azidated 1-palmitoyl-2-oleoylphosphatidylethanolamine
[0071] Chloroethylamine hydrochloride (5 g, 43.1 mmol) and sodium azide (8.4 g, 129 mmol) were dissolved in 30 mL of water, and reacted at 80° C. for 15 h. After the reaction, solid potassium hydroxide was added to adjust the pH of the reaction solution to 12-14, extracted with ether, and the organic layer was concentrated to obtain a light yellow oil (2.8 g, 75.6%). The light yellow oil (2.8g, 32mmol) and hexahydrophthalic anhydride (4.16g, 27mmol) were dissolved in 50mL of chloroform, reacted at 25°C for 5h, and subjected to dichloromethane / methanol column chromatography to obtain a white powdery solid (hexaazide hydrobenzoic acid, 5.9 g, 90.9%). Dissolve hexahydrobenzoic acid azide (0.33g, 1.39mmol) and N-hydroxysuccinimide (0.32g, 2.78mmol) in 20mL of chloroform, add 20mL of 1-ethyl-(3-bis Methylaminopropyl) carbodiimide hydrochloride (0.53g, 2.78mmol), activated for 1h, adding 1-palmitoyl-2-ol...
Embodiment 3
[0074] Preparation of Cholesterol Azide
[0075] Chloroethylamine hydrochloride (5 g, 43.1 mmol) and sodium azide (8.4 g, 129 mmol) were dissolved in 30 mL of water, and reacted at 80° C. for 15 h. After the reaction, solid potassium hydroxide was added to adjust the pH of the reaction solution to 12-14, extracted with ether, and the organic layer was concentrated to obtain a light yellow oil (2.8 g, 75.6%). The light yellow oil (2.8g, 32mmol) and hexahydrophthalic anhydride (4.16g, 27mmol) were dissolved in 50mL of chloroform, reacted at 25°C for 5h, and subjected to dichloromethane / methanol column chromatography to obtain a white powdery solid (hexaazide hydrobenzoic acid, 5.9 g, 90.9%). Dissolve hexahydrobenzoic acid azide (2.65g, 11mmol) and lutamine (0.27g, 2.2mmol) in 30mL of chloroform, add 30mL of 1-ethyl-(3-dimethylaminopropyl) dropwise in an ice bath Base) carbodiimide hydrochloride (4.2g, 22mmol), activated for 1h, added cholesterol (2.13g, 5.5mmol) and reacted at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com