9,10-diarylethene anthracene (CPASA) aggregation-induced light emitting molecule and preparation method thereof
A diaryl vinyl anthracene, aggregation-induced luminescence technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as unfavorable fluorescence emission, application limitations of fluorescent molecules, and weakened fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
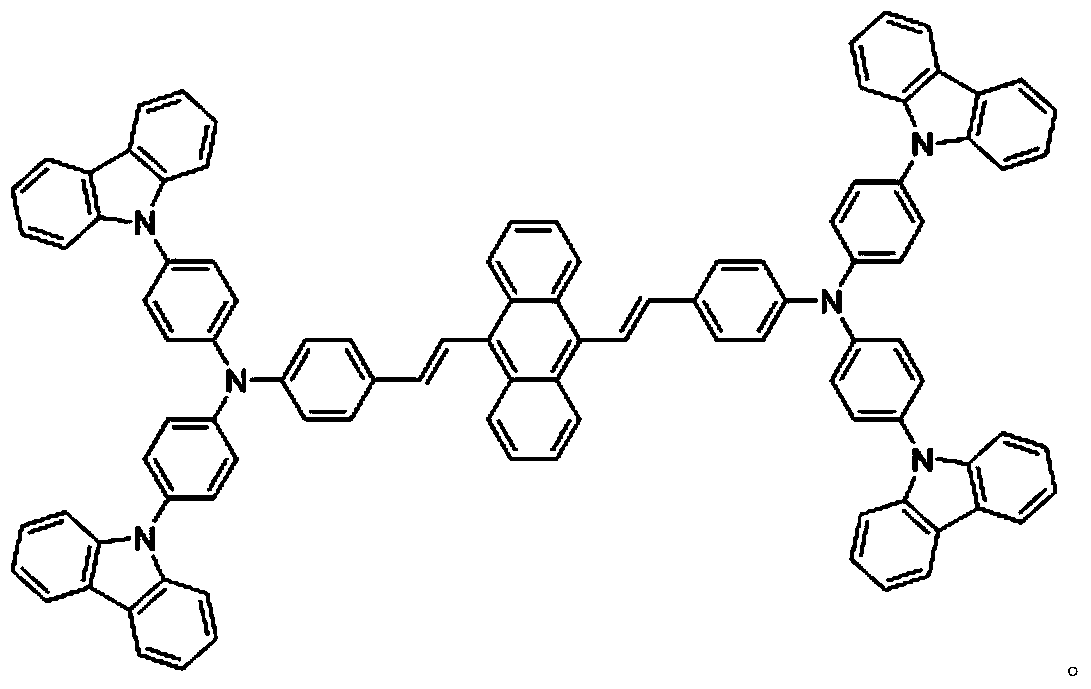
[0017] Example 1. 9,10-diarylvinylanthracene aggregation-induced luminescent molecule 9,10-bis{4-{N,N-bis[4-(carbazol-9-yl)phenyl]amino}styryl} The preparation method of anthracene CPASA
[0018] (1) 9,10-bis{4 -[N,N-bis(4-iodophenyl)amino]styryl}anthracene (SM), the synthetic route is as follows:
[0019]
[0020] For the specific preparation method, refer to a pyridine-triphenylamine-anthracene conjugated molecule with aggregation-induced luminescent properties and its preparation publication No. CN103524404A Example 1.
[0021] (2) 9,10-bis{4-[N,N-bis(4-iodophenyl)amino]styryl}anthracene (SM) reacts with carbazole at a molar ratio of 1:6 to obtain 9,10- Bis{4-{N,N-bis[4-(carbazol-9-yl)phenyl]amino}styryl}anthracene (CPASA), the synthetic route is as follows:
[0022]
[0023] The specific preparation method is: under anhydrous and oxygen-free conditions, weigh 1.00g (5.98mmol) of carbazole and dissolve it in 10mL of N,N-dimethylacetamide, add 0.78g (4.01mmol) of cu...
Embodiment 2
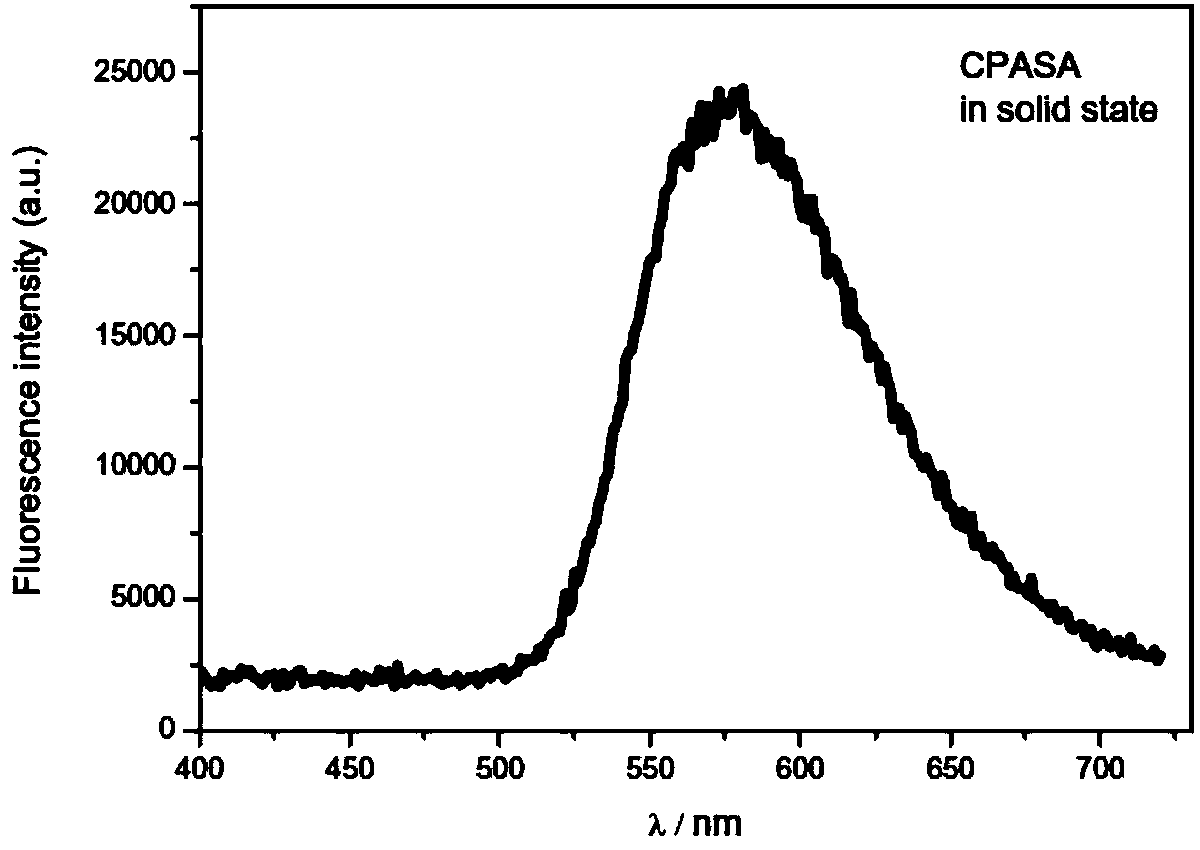
[0029] Example 2.9,10-Diarylvinylanthracene CPASA solid-state fluorescence
[0030] 9,10-Diarylvinylanthracene CPASA emits yellow light in solid state with a maximum emission wavelength of 581nm. The solid state of CPASA emits bright yellow fluorescence under ultraviolet light in a dark room. attached figure 1 Fluorescence of 9,10-bis{4-{N,N-bis[4-(carbazol-9-yl)phenyl]amino}styryl}anthracene (CPASA) solid powder prepared for Example 1 of the present invention spectrum.
Embodiment 3
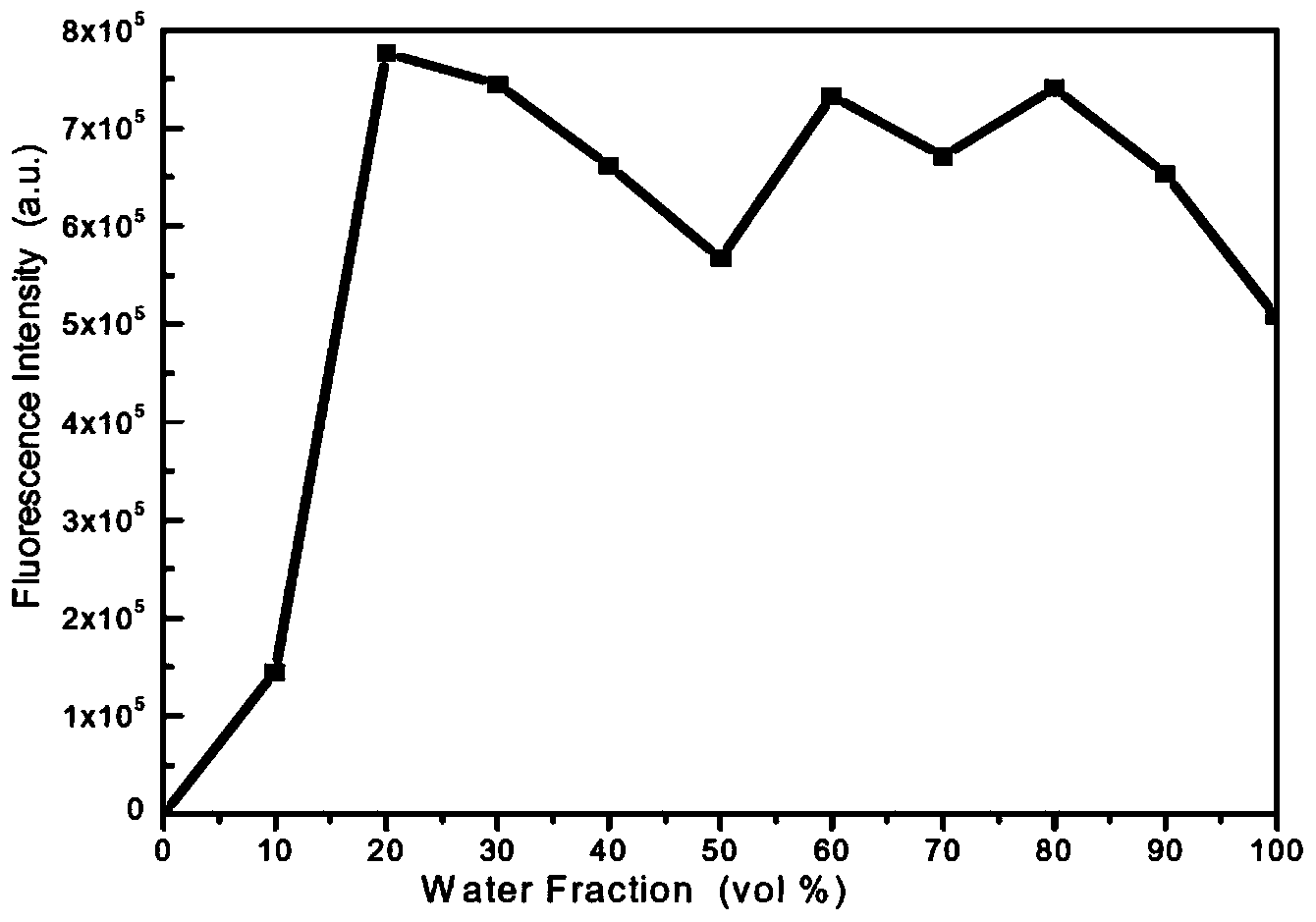
[0031] Example 3.9, Aggregation-induced fluorescence enhancement of 10-diarylvinylanthracene CPASA
[0032] 9,10-Diarylvinylanthracene CPASA is insoluble in water, but soluble in organic solvents such as dimethylformamide, tetrahydrofuran, chloroform, dichloromethane, ethyl acetate, and acetone. It does not emit light in organic solvents such as dimethylformamide, and has no fluorescence under ultraviolet light in a dark room, but the fluorescence increases rapidly after adding 20% water by volume, and emits bright yellow fluorescence. attached figure 2 9,10-bis{4-{N,N-bis[4-(carbazol-9-yl)phenyl]amino}styryl}anthracene (CPASA) prepared in Example 1 of the present invention in water / di Fluorescence emission intensity changes in methylformamide mixed solutions with different water contents.
[0033] The 9,10-diarylvinylanthracene CPASA of the present invention has aggregation-induced luminescent properties, and has important application value in the fields of fluorescent s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com