A kind of polyisourea polymer and its preparation method and application
A technology of polymer and polymerization reaction, which is applied in fluorescence/phosphorescence, instruments, analytical materials, etc., and can solve problems such as high-efficiency reaction and unmanned research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
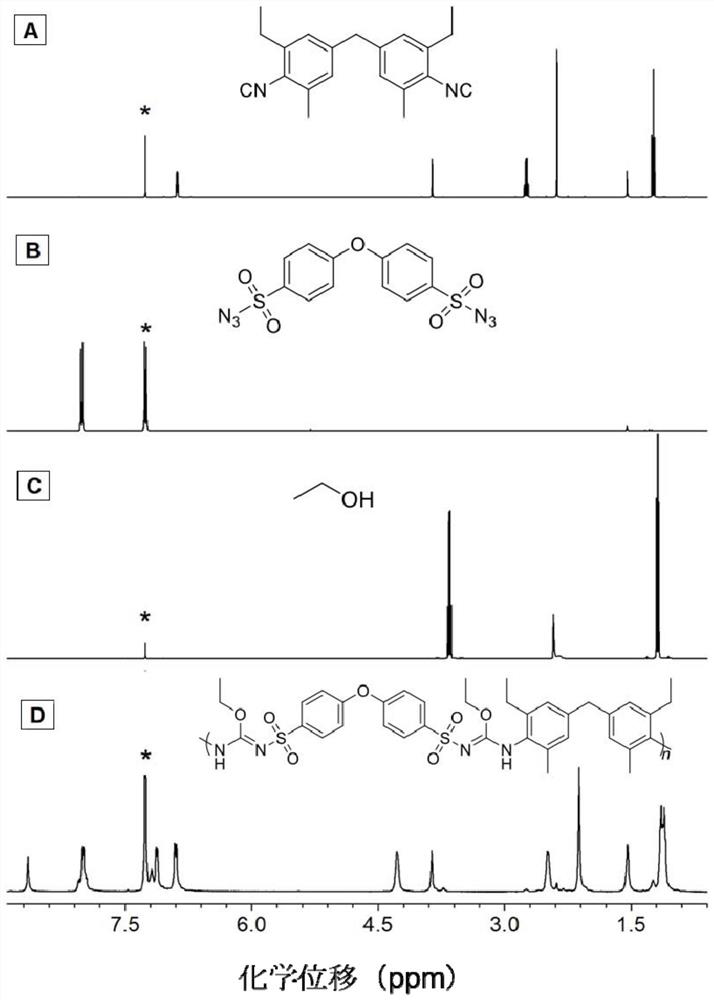
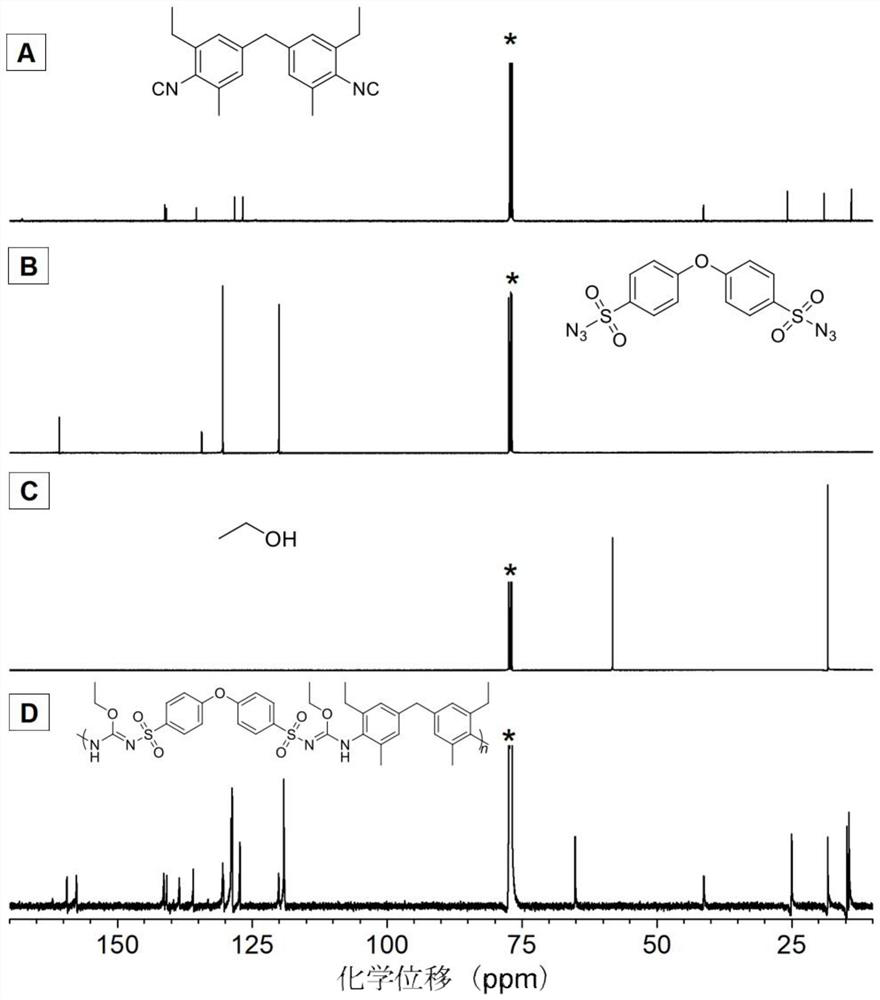
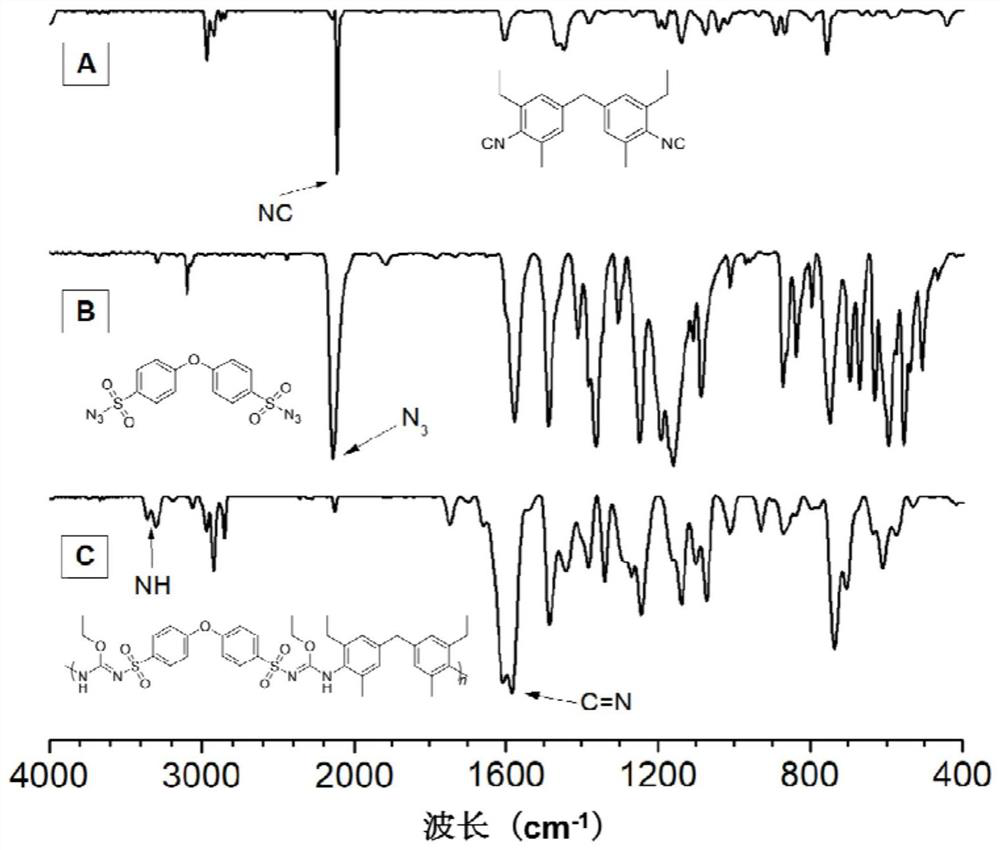
[0046] A polyisourea polymer, its structural formula is as shown in P1:
[0047]
[0048] n is an integer from 2 to 200;
[0049] The polyisourea polymer is prepared by multi-component polymerization reaction of isonitrile monomer, azide monomer and alcohol monomer, and the reaction equation is as formula (1):
[0050]
[0051] Wherein, the synthesis method of monomer M1 can be synthesized according to the synthesis method of the applicant in the published literature (Zakrzewski J., Krawczyk M.. Phosphorus, Sulfur, and Silicon, 2009, 184(7): 1880-1903.); The synthetic method of M2 can be synthesized according to the synthetic method of the applicant in the published literature (Alan R.K., James W.R., Rachel M.W..J.Environ.Eng., 2007,25:79-109.); M3 is ethanol, It can be purchased from the market, and in this example, it was purchased from Sigma-Aldrich Company.
[0052] The preparation steps of described polyisourea polymer are as follows:
[0053] Add 60.5mg (0.2mmol...
Embodiment 2
[0057] A polyisourea polymer, its structural formula is as shown in P2:
[0058]
[0059] n is an integer from 2 to 200;
[0060] The polyisourea polymer is prepared by multi-component polymerization reaction of isonitrile monomer, azide monomer and alcohol monomer, and the reaction equation is as formula (2):
[0061]
[0062] Wherein, the synthesis method of the monomer M1 is the same as that of Example 1; the synthesis method of the monomer M4 is the same as that of Example 1; M3 is ethanol, which can be purchased from the market, and is purchased from Sigma-Aldrich in this embodiment.
[0063] The preparation steps of described polyisourea polymer are as follows:
[0064] Add 60.5mg (0.2mmol) monomer M1, 72.9mg (0.2mmol) monomer M4, 115.2mg (0.5mmol) monomer M3 and 2.9mg (0.02mmol) catalyst CoC into a 10mL polymerization tube 2 o 4 , inject 0.67mL of N,N-dimethylformamide solvent or 0.67mL of monomer M3 with a syringe, react at 80°C for 2h, and rotate at 500 rpm. ...
Embodiment 3
[0067] A polyisourea polymer, its structural formula is as shown in P3:
[0068]
[0069] n is an integer from 2 to 200;
[0070] The polyisourea polymer is prepared by multi-component polymerization reaction of isonitrile monomer, azide monomer and alcohol monomer, and the reaction equation is as formula (3):
[0071]
[0072] Wherein, the synthesis method of monomer M5 is the same as in Example 1; the synthesis method of monomer M2 is the same as in Example 1; M3 is ethanol, which can be purchased from the market, and in this embodiment, it is purchased from Sigma-Aldrich Company.
[0073] The preparation steps of described polyisourea polymer are as follows:
[0074] Add 27.2mg (0.2mmol) monomer M5, 76.1mg (0.2mmol) monomer M2, 115.2mg (0.5mmol) monomer M3 and 2.9mg (0.02mmol) catalyst CoC in a 10mL polymerization tube 2 o 4 , inject 0.67mL of N,N-dimethylformamide solvent or 0.67mL of monomer M3 with a syringe, react at 80°C for 2h, and rotate at 500 rpm. After t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com