A kind of carbazole-based triphenylamine-oxadiazole fluorescent molecule and its preparation method and application
A carbazolyl triphenylamine and fluorescent molecule technology, applied in the field of fluorescent molecules, can solve problems such as fluorescence quenching, and achieve the effects of high stability, good solubility, and good aggregation-induced luminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of the above-mentioned carbazolyltriphenylamine-oxadiazole fluorescent molecule comprises the following steps: in tetrahydrofuran solution, compound M 1 Under the catalysis of NaH and compound M 2 Carry out Wittig reaction, make compound TM namely described carbazolyltriphenylamine-oxadiazole fluorescent molecule, synthetic route is as follows:
[0025]
Embodiment 1
[0034] Embodiment 1 Preparation of carbazolyl triphenylamine-oxadiazole fluorescent molecule
[0035] 0°C and under nitrogen protection, 1.00g (1.58mmol) of compound M 2 Dissolve in 20mL of tetrahydrofuran (THF) solution, and slowly add 0.06g (2.68mmol) sodium hydride suspension in 5mL THF dropwise. Stir at 0°C for 1 h, slowly dropwise add 1.44 g (1.74 mmol) of compound M 1 20mL THF solution. The temperature was gradually raised to 80°C under stirring, and the reaction was carried out for 2 hours. After the reaction, the reaction solution was cooled to room temperature and poured into water. Solids were precipitated, filtered by suction, dried, purified by column chromatography and recrystallization to obtain TM as a yellow solid with a yield of 50%.
[0036] Analysis of the resulting compound TM:
[0037] 1 HNMR (CDCl 3 ,ppm): δ8.20(s,4H),8.19(d,J=9.43Hz,2H),8.14(d,J=8.37Hz,2H),7.72(d,J=8.25Hz,2H),7.61 -7.58(m,4H),7.58-7.55(m,4H),7.55-7.53(m,4H),7.49-7.44(m,8H),7.36(d,...
Embodiment 2
[0042] Example 2 Aggregation-induced Luminescent Properties of Carbazolyl Triphenylamine-Oxadiazole Fluorescent Molecules
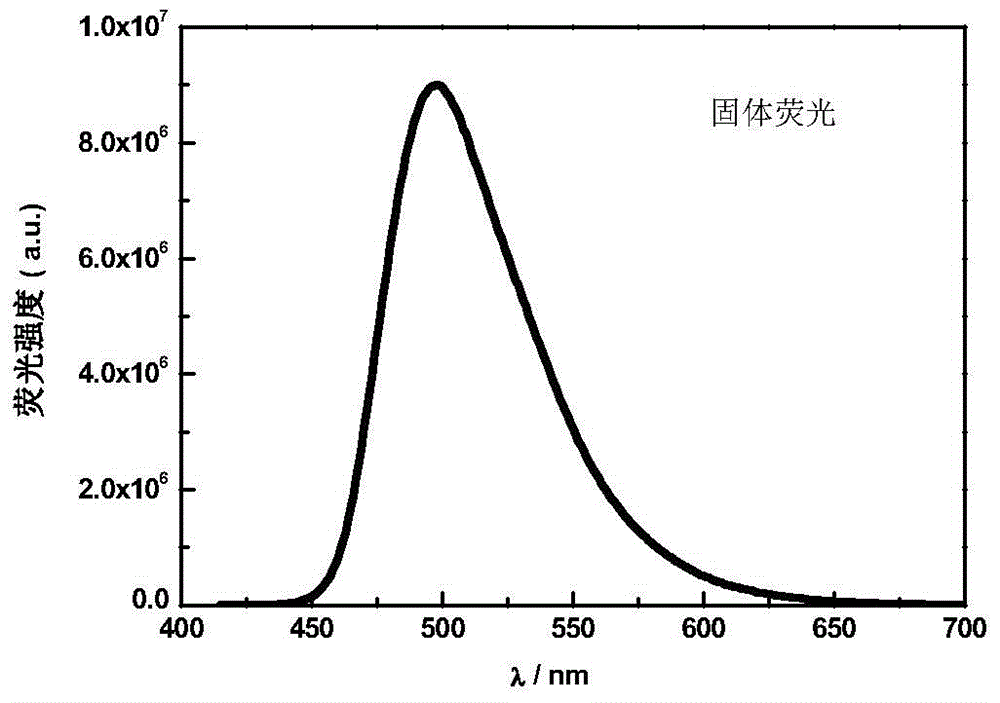
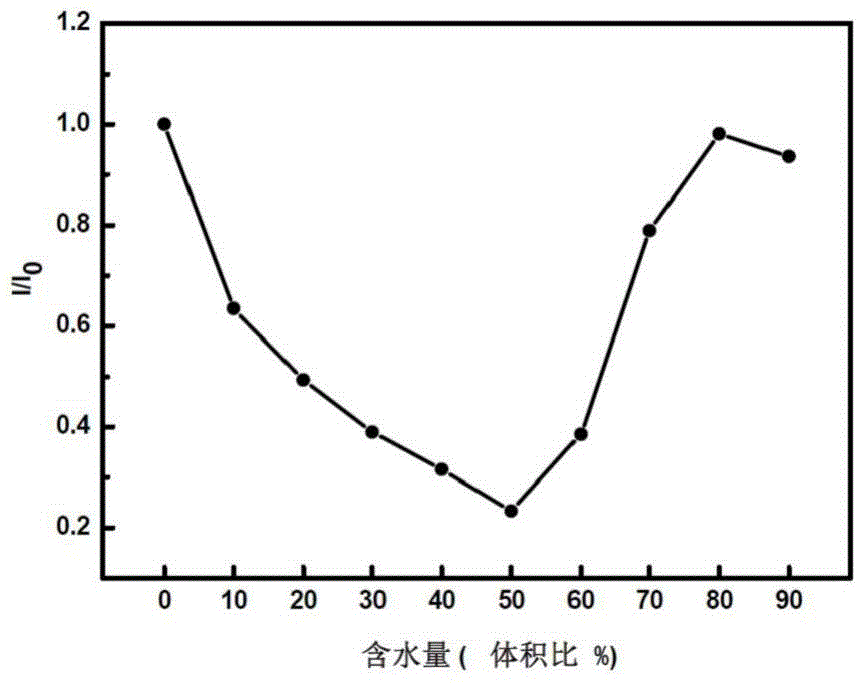
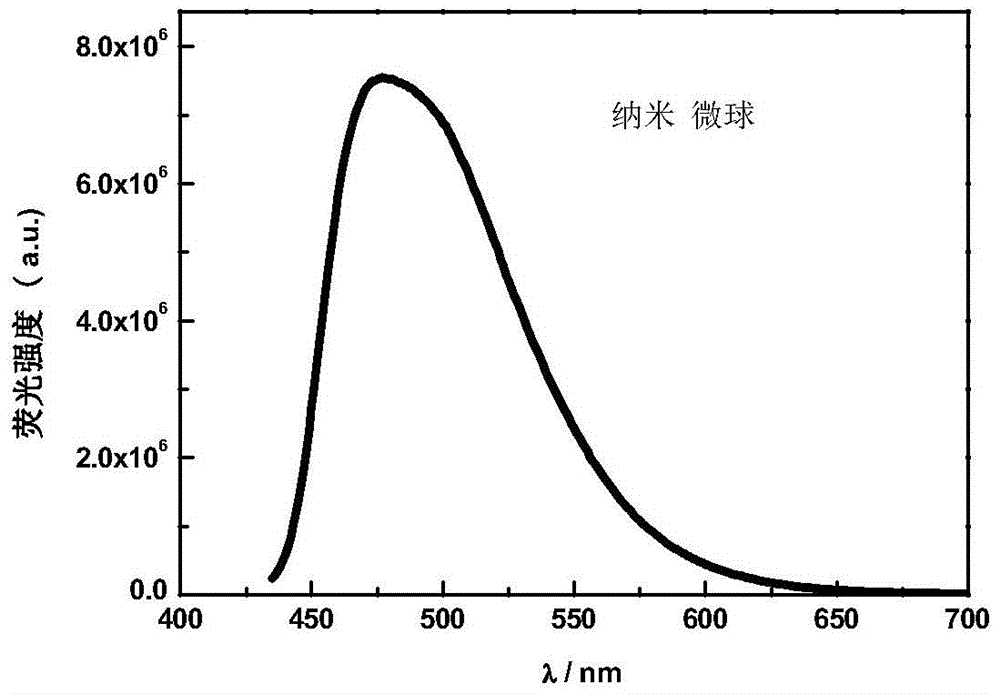
[0043] figure 1 It is the solid-state fluorescence spectrum of the carbazolyltriphenylamine-oxadiazole fluorescent molecule TM. The solid powder of the fluorescent molecule TM in the dark room emits bright green fluorescence under the ultraviolet lamp, and the maximum emission wavelength is 498nm. Carbazolyltriphenylamine-oxadiazole fluorescent molecule TM is insoluble in water, but soluble in organic solvent tetrahydrofuran, and the fluorescence intensity changes with the water content in the water / tetrahydrofuran mixed solution. figure 2 It is the change trend diagram of the fluorescence relative intensity of the carbazolyltriphenylamine-oxadiazole fluorescent molecule TM in the water / tetrahydrofuran mixed solution with different water contents. When the water content increased from 0 to 50%, the fluorescence intensity gradually weakened. When the wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com