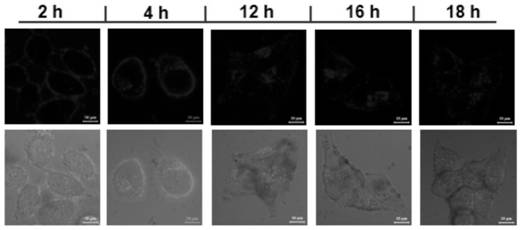
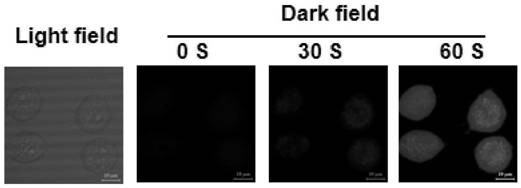
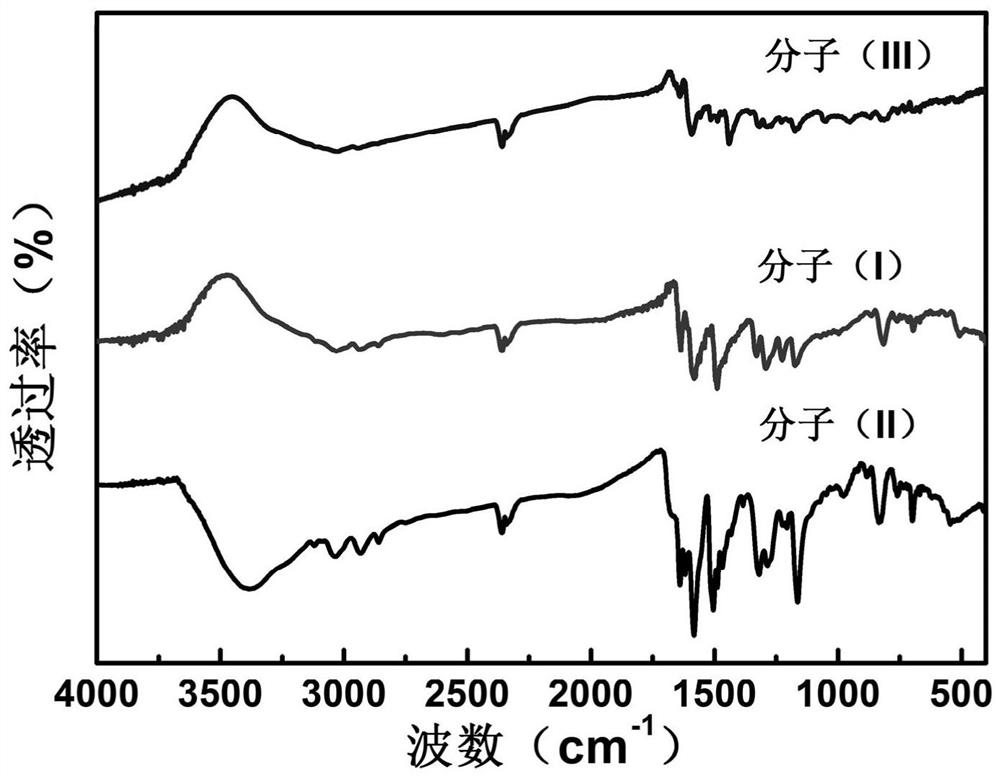
Triphenylamine-quaternary ammonium salt type polymer as well as preparation method and application thereof
A triphenylamine and polymer technology, applied in the field of triphenylamine-quaternary ammonium salt polymer and its preparation, can solve the problems of poor biocompatibility and short emission wavelength, and achieve long emission wavelength, deep penetration depth, and damage small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kind of triphenylamine-quaternary ammonium salt type polymer, its structural formula is shown in following formula (I):
[0047]
[0048] The preparation method of described triphenylamine-quaternary ammonium salt type polymer is as follows:
[0049] 4,4'-bis(4-pyridyl)triphenylamine (0.04g, 0.1mmol), 1,6-dibromohexane (0.024g, 0.1mmol), and 20mL tetrahydrofuran were added to a 50ml round bottom flask in sequence. After stirring and reacting at 85°C for 48 hours, the solvent was distilled off under reduced pressure, and the filter residue was washed successively with tetrahydrofuran and diethyl ether to obtain 0.048 g of an orange-yellow solid (65% yield). The degree of polymerization of the polymer obtained in this embodiment is 1-15.
Embodiment 2
[0051] A kind of triphenylamine-quaternary ammonium salt type polymer, its structural formula is shown in following formula (II):
[0052]
[0053] The preparation method of described triphenylamine-quaternary ammonium salt type polymer is as follows:
[0054] Mix N,N-bis(4-formylphenyl)aniline (0.03g, 0.1mmol) with 1,1'-(hexane-1,6-diyl)bis(4-methyl Pyridin-1-ium) bromide (0.043g, 0.1mmol), a few drops of piperidine and 20mL of ethanol were added into a 50mL round bottom flask, heated at 90°C under reflux and stirred for 4 hours. After the reaction was completed, it was cooled, distilled under reduced pressure, and washed successively with tetrahydrofuran and diethyl ether to obtain 0.062 g of a red solid (yield 86%). The degree of polymerization of the polymer obtained in this embodiment is 1-10.
Embodiment 3
[0056] A kind of triphenylamine-quaternary ammonium salt type polymer, its structural formula is shown in following formula (III):
[0057]
[0058] The preparation method of described triphenylamine-quaternary ammonium salt type polymer is as follows:
[0059] Combine 5,5'-((phenylazadiyl)bis(4,1-phenylene))bis(thiophene-2-carbaldehyde) (0.046g, 0.1mmol) with 1,1 prepared in step S2 Add '-(hexane-1,6-diyl)bis(4-methylpyridin-1-ium) bromide (0.043 g, 0.1 mmol), a few drops of piperidine and 20 mL of ethanol to a 50 mL round bottom flask , heated at reflux and stirred at 90°C for 4 hours; cooled after the reaction, distilled under reduced pressure, and washed with tetrahydrofuran and diethyl ether in sequence to obtain 0.075 g of a dark red solid (yield 85%). The degree of polymerization of the polymer obtained in this embodiment is 1-14.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com