Chemically modified thymopentin and synthetic method thereof
A technology of chemical modification and synthesis method, which is applied in the field of biochemical drug synthesis, can solve the problems of high molecular weight of PEG, difficulty in obtaining modified site products, and decreased biological activity of drugs, so as to achieve high bioavailability, overcome repeated repeated and long-term Injection, the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthetic method of the Thymopentin of embodiment 1 chemical modification
[0035] Include the following steps:
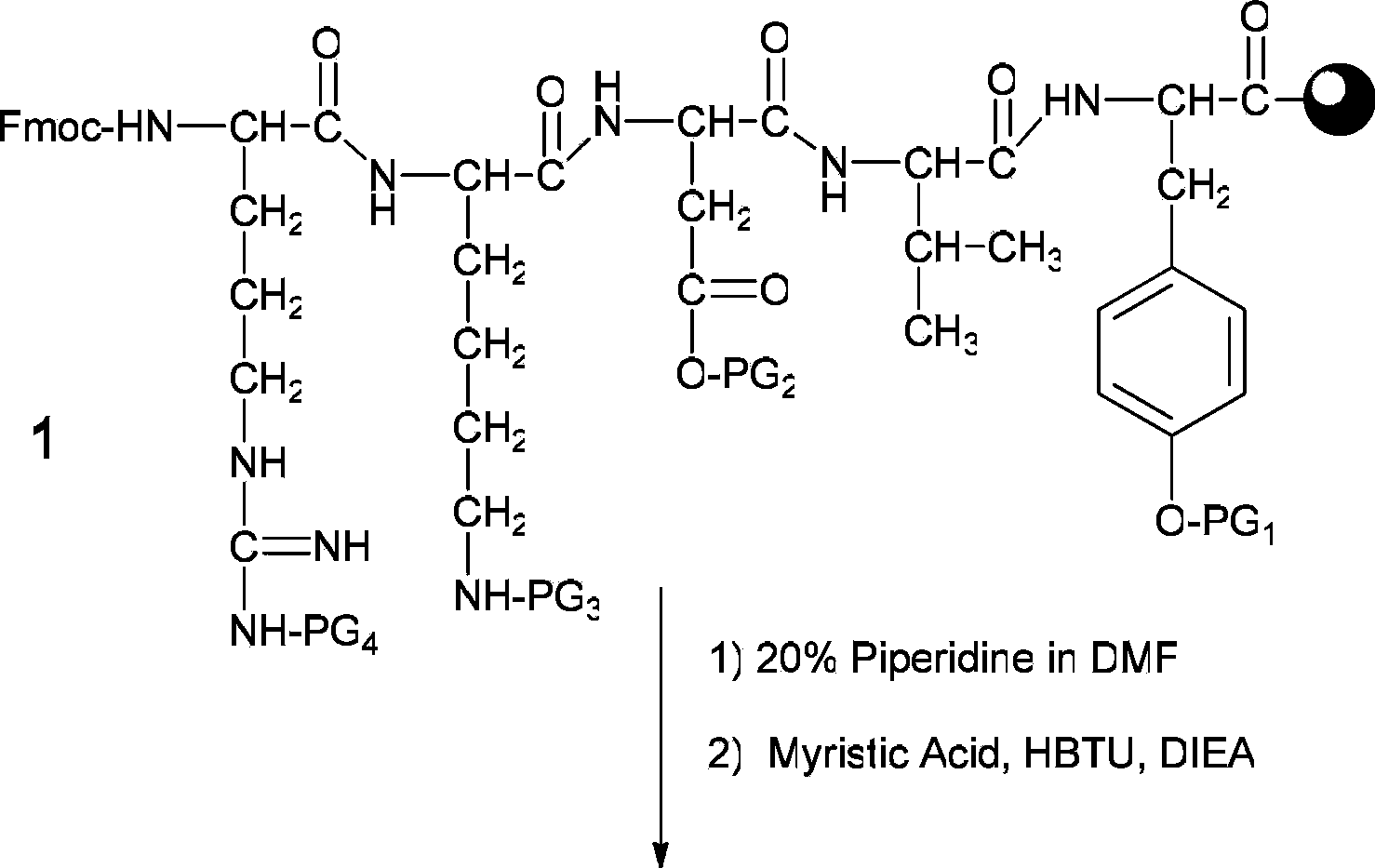
[0036] (1) Weigh 200 mg of Fmoc-protected tyrosine king resin (Fmoc-Tyr(tBu)-Wang Resin
[0037] 0.25 mmol / g) in a manual solid-phase peptide synthesizer, adding DCM (dichloromethane) to swell for 30 minutes. Sequentially with Fmoc-Val-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Lys(Boc)-OH and
[0038]Fmoc-Arg(Pbf)-OH is the amino acid raw material, with benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA) As a polypeptide condensing agent, the equivalent ratio of the resin to each protected amino acid and polypeptide condensing agent is Tyrosine King Resin: Amino Acid: HBTU: DIEA=1: 3: 3: 6, according to the standard Fmoc strategy to synthesize Fmoc-protected loaded thymus Wang resin of the pentapeptide (compound 1).
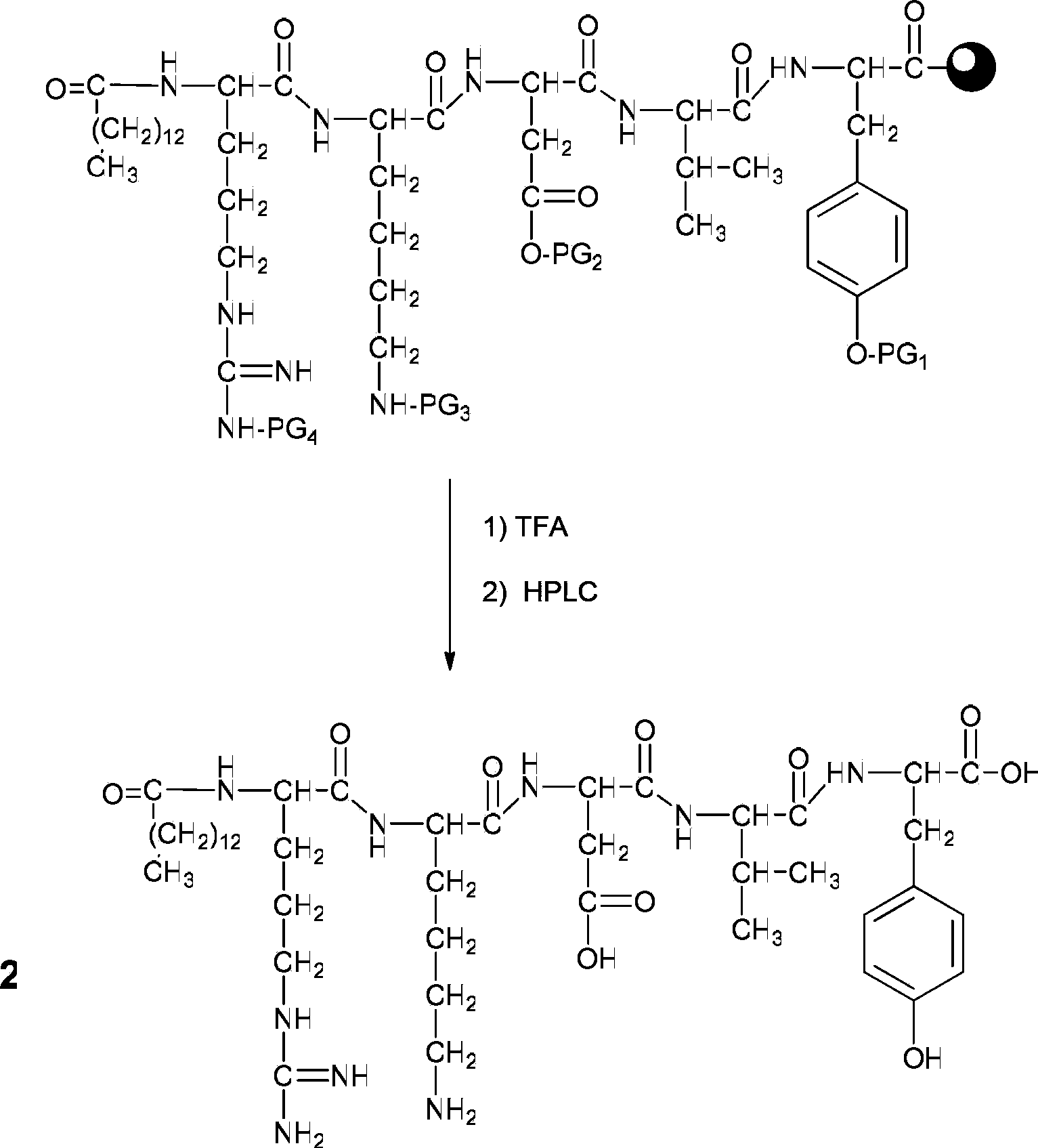
[0039] (2) After removing the terminal amino Fmoc protecting group with 2 ml of piperidine deprot...
Embodiment 2
[0044] The synthetic method of the Thymopentin of embodiment 2 chemical modifications
[0045] Include the following steps:
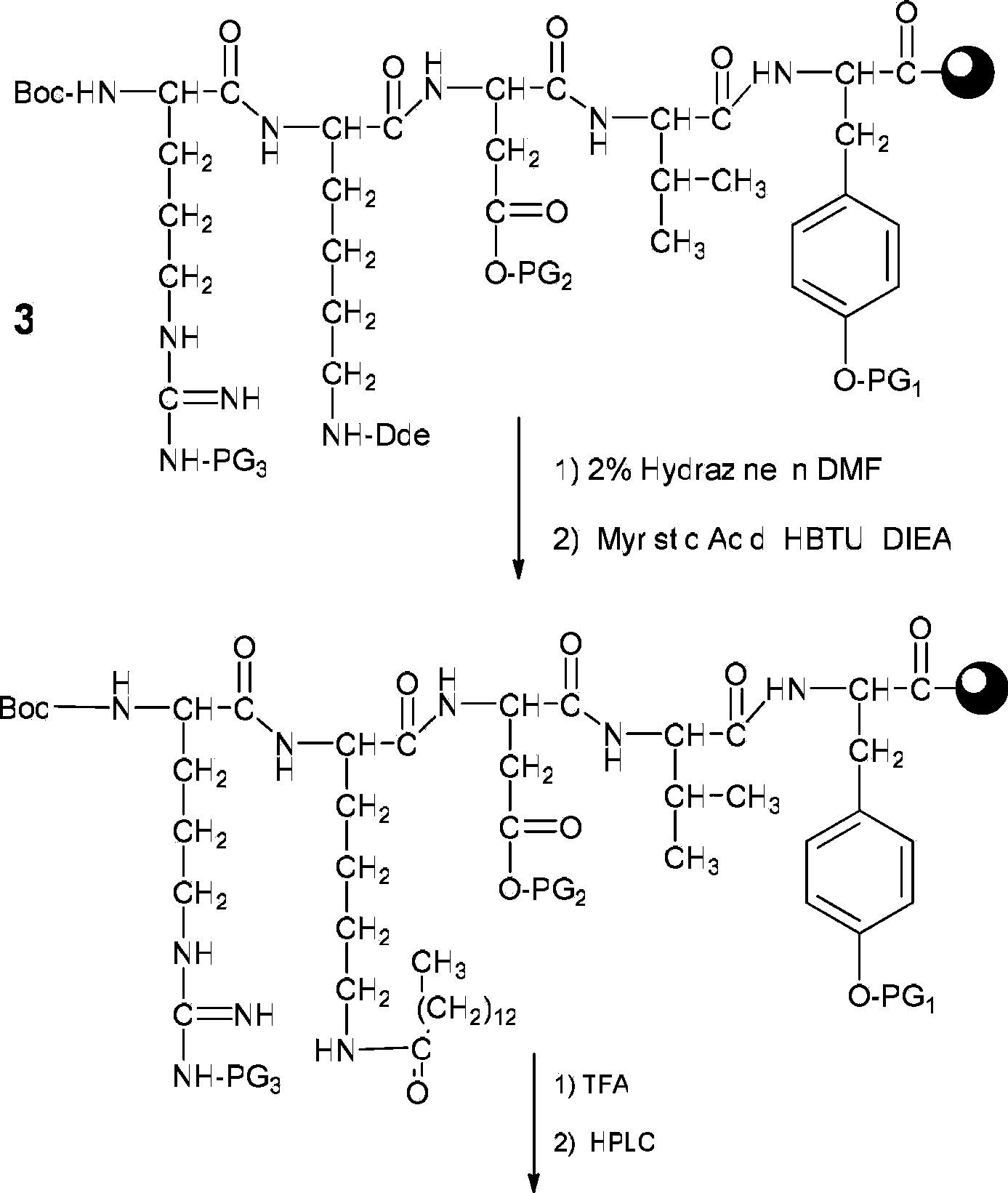
[0046] (1) Weigh 200 mg of Fmoc-protected tyrosine king resin (Fmoc-Tyr(tBu)-Wang Resin
[0047] 0.25 mmol / g) in a manual solid-phase peptide synthesizer, adding DCM (dichloromethane) to swell for 30 minutes.
[0048] Sequentially with Fmoc-Val-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Lys(Dde)-OH and
[0049] Boc-Arg(Pbf)-OH is used as amino acid raw material, with benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA) As a polypeptide condensing agent, the equivalent ratio of the resin to each protected amino acid and polypeptide condensing agent is Tyrosine King Resin: Amino Acid: HBTU: DIEA=1: 3: 3: 6, and Thymopentin is synthesized according to the standard Fmoc strategy. Wang resin (compound 3).
[0050] (2) After reacting 2% dimethylformamide (DMF) solution at room temperature with the volume percentage of 2m...
Embodiment 3
[0056] The synthetic method of the Thymopentin of embodiment 3 chemical modifications
[0057] Include the following steps:
[0058] (1) Myristic acid (460 mg, 2mmol) was dissolved in 100 ml of dichloromethane (DCM), activated with equivalent HBTU (759 mg, 2mmol) and DIEA (260 mg, 2mmol) for 2 minutes,
[0059] Add Fmoc-Lys-OH (736 mg, 2 mmol), react at room temperature for 1 hour, evaporate DCM under reduced pressure, and purify the crude product by HPLC to obtain Fmoc-protected myristic acid-modified side chain lysine
[0060] (Fmoc-Lys(Myr)-OH) 872 mg, yield 76%.
[0061] The reaction equation is as follows:
[0062]
[0063] (2) Weigh 200 mg of Fmoc-protected tyrosine king resin (Fmoc-Tyr(tBu)-Wang Resin
[0064] 0.25 mmol / g) in a manual solid-phase peptide synthesizer, adding DCM (dichloromethane) to swell for 30 minutes.
[0065] Sequentially with Fmoc-Val-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Lys(Myr)-OH and
[0066] Boc-Arg(Pbf)-OH is used as amino acid raw material, wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com