Method for improving expression quantity of pichia pastoris foreign protein by using mercaptan peroxidase
A thiol peroxide, protein expression technology, applied in the direction of using vectors to introduce foreign genetic material, recombinant DNA technology, etc., can solve problems such as low expression efficiency and insufficient target protein expression, reduce the impact of the response mechanism, overcome the Free radical accumulation, effects that contribute to correct conformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1: Overexpression of thiol peroxidase gene in methanol-inducible Pichia pastoris of exogenous recombinant protein.
[0011] Taking the Pichia pastoris GAP constitutive promoter expression vector to overexpress the Pichia thiol peroxidase gene as an example: design primers, clone the thiol peroxidase gene Tpx fragment from Pichia pastoris, and Komagataella published by NCBI Compared with the thiol peroxidase gene sequence of pastoris GS115 (PAS_chr2-2_0382), homology analysis showed that the homology between the cloned gene fragment and the gene bank Komagataella pastoris GS115 thiol peroxidase gene was 100%.
[0012] The primer sequences are:
[0013] Tpx-F(EcoRI):5'CAGGAATTCATGTCTTCATTTTATGATCTGGCCCCATTA3'
[0014] Tpx-R(XbaI): 5'GCTCTAGATTACAACTGGTTTGCAGGTGGAAAATGTT3'
[0015] The cloned Tpx gene fragment and pGAPZB, a commonly used expression vector of Pichia pastoris, were digested with EcoRI and XbaI and ligated with T4 DNA ligase overnight at 16°C. The l...
Embodiment 2
[0017] Example 2: Fermentation of exogenous protein expression-enhanced strains overexpressing Tpx gene.
[0018] Medium: the seed and slant medium are yeast basic fermentation medium is BMGY medium (1L): peptone 20g, yeast extract 10g, glycerol 10%, YNB13.4g, 100mM phosphate buffer (pH6.0); induction The medium is BMMY medium (1L): 20g peptone, 10g yeast extract, 20g glucose; 20g agar added to solid medium; 13.4g YNB, 100mM phosphate buffer (pH6.0); 1% methanol, supplemented with methanol induction Add time interval is 24h.
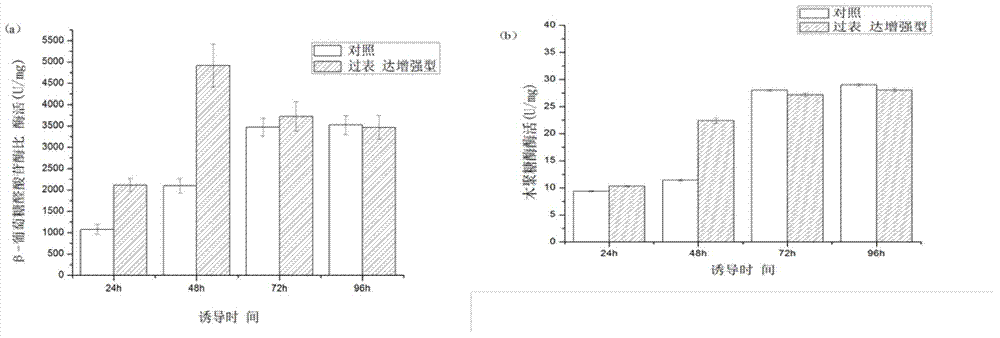
[0019] Culture method: select Pichia pastoris positive transformants expressing β-glucuronidase and xylanase overexpressing thiol peroxidase gene, and inoculate them into YPD seed medium. The seeds cultivated at 30°C and 200rpm until the OD600 is between 1.6-1.7 are transferred to the basic fermentation medium with a 2% inoculation amount, and at 30°C and 200rpm; induction conditions: cultivated in BMGY until the OD value is 1.2- At 1.5, the yeast cell...
Embodiment 3
[0022] Example 3: The thiol peroxidase gene regulates the expression level of exogenous recombinant protein at the transcriptional level.
[0023] Check the thiol peroxidase gene sequence of several functionally related or similar sequences derived from Pichia pastoris or Saccharomyces cerevisiae and other species through the NCBI database, design primers, and design with OE-PCR or restriction site design. Constitutive and inducible promoter expression plasmids of different strengths from Pichia pastoris or Saccharomyces cerevisiae are connected, and methanol-inducible Pichia pastoris transformed into exogenous recombinant protein is co-expressed or overexpressed, and the promoter strength and induction method Regulate the level of thiol peroxidase gene transcription, and then affect the secretion and expression of recombinant protein in methanol-inducible Pichia pastoris engineering bacteria. The enzyme activity of exogenous protein expressed in strains with low copy of thiol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com