Method for detecting macromolecular substance in reduning injection
A technology for polymer substances and injections, which is used in measurement devices, material separation, and analysis of materials. High and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
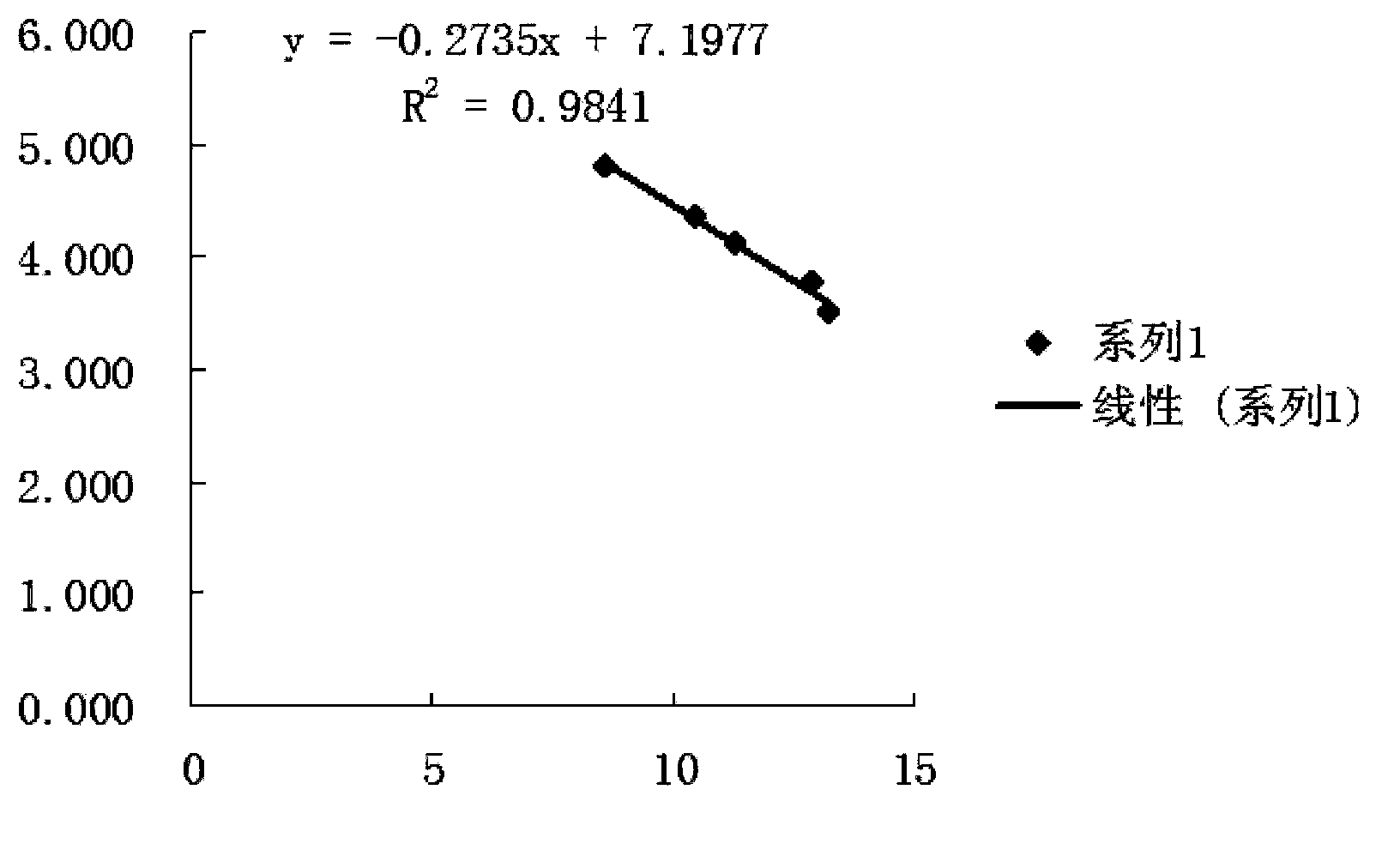
[0040] Embodiment 1 takes protein as the establishment of the reference substance HPLC method
[0041] 1.1 Optimal chromatographic conditions
[0042] 1.1.1 Determination of detection wavelength
[0043] Because proteins have better absorption at low wavelengths, 214nm was selected as the detection wavelength.
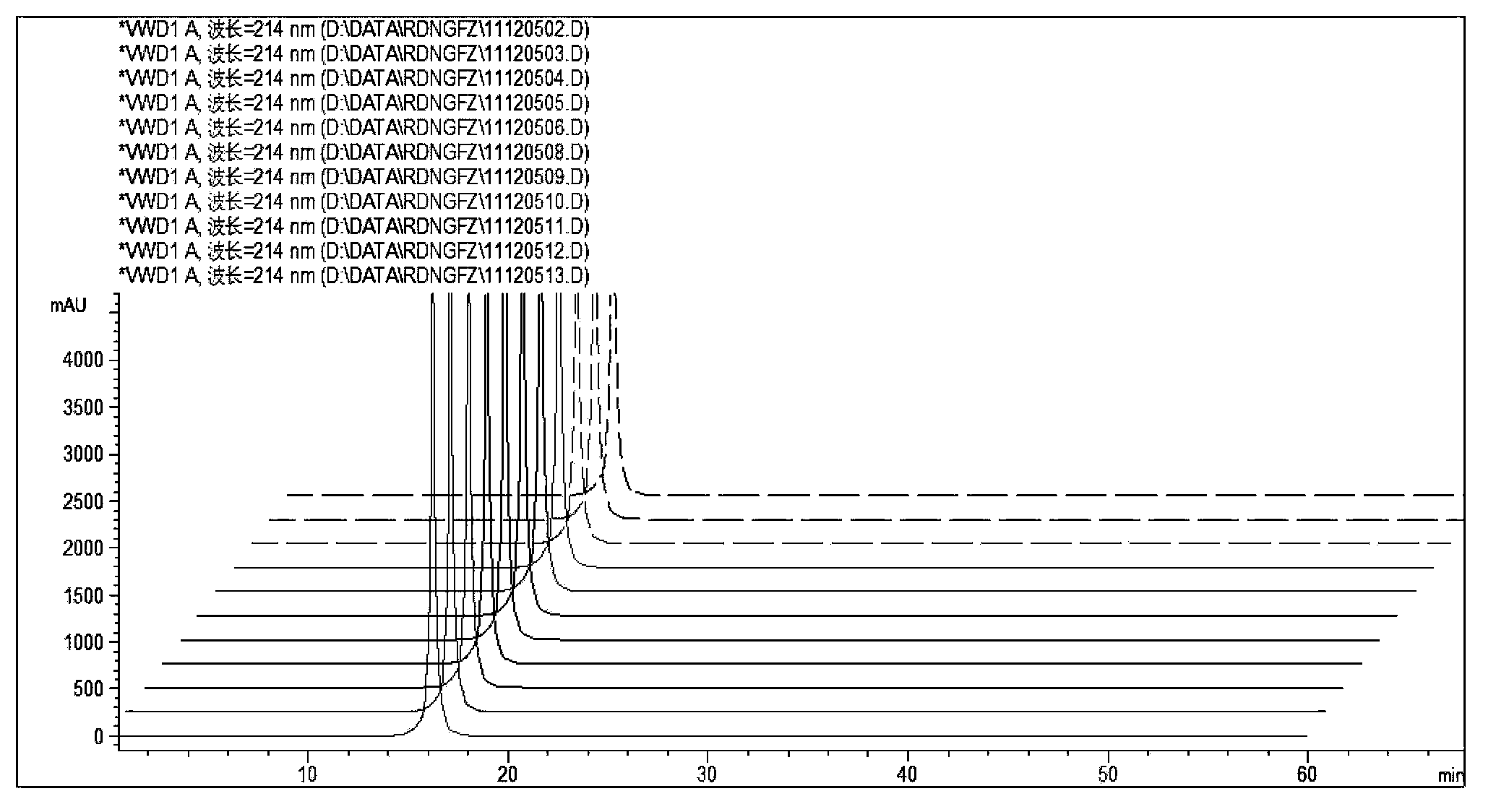
[0044] 1.1.2 mobile phase selection
[0045] Instrument: Agilent1100 (VWD);
[0046] Gel column: TSK G2000SWxl, 300mm×7.8mm, 5μm;
[0047] Column temperature: 30°C;
[0048] Detection wavelength: 214nm;
[0049] Mobile phase one: use 0.1mol / L sodium sulfate as the mobile phase, and the flow rate is 0.5ml / min;
[0050] Mobile phase two: use acetonitrile-0.05% trifluoroacetic acid (the volume ratio of acetonitrile and 0.05% trifluoroacetic acid is 30:70) as the mobile phase, and the flow rate is 0.7ml / min;
[0051] Mobile phase three: use acetonitrile-0.08% trifluoroacetic acid (the volume ratio of acetonitrile and 0.08% trifluoroacetic acid is 30:70) as the mobil...
Embodiment 2
[0094] Example 2 Determination of macromolecular substances
[0095] 2.1 Determination method Determination method of macromolecular substances in Reduning Injection—Tymosin α1 as reference substance
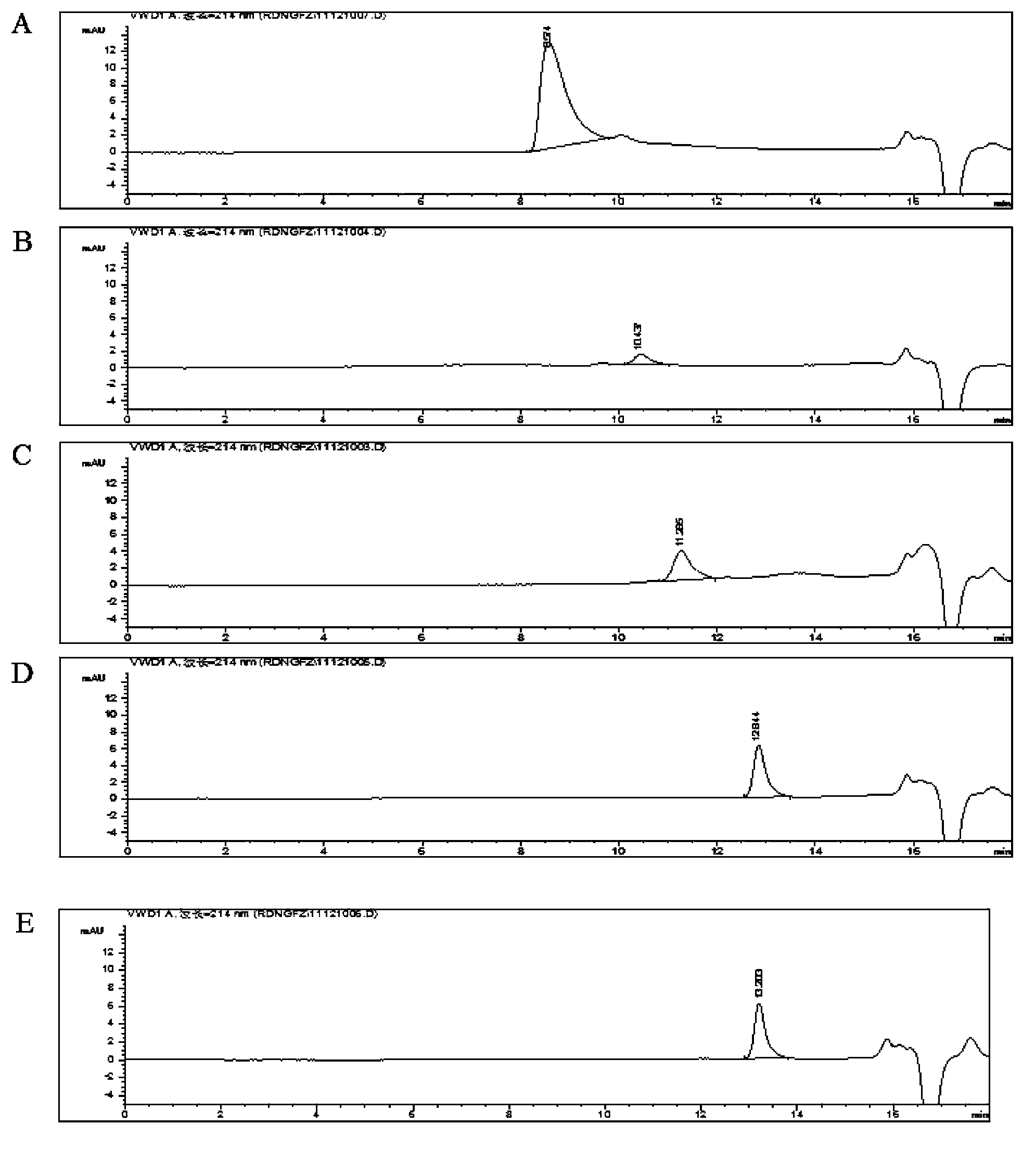
[0096] Precisely measure 1ml of Reduning injection, add mobile phase to dilute to 10ml, shake well, and use it as the test solution; take an appropriate amount of thymosin α1 standard substance, weigh it, dissolve and dilute with water to make a solution containing 0.025mg per 1ml As a reference solution. Precisely measure 10 μl each of the test solution and the reference solution and inject it into the liquid chromatograph, record the chromatogram, regard the peak that is prior to the peak time of thymosin α1 (molecular weight 3108) as a polymer substance, and calculate it by the area normalization method Its content, macromolecular substances shall not exceed 0.05%.
[0097] Chromatographic conditions and system suitability test chromatographic column: TSK G2000SWXL (300mm×7...
Embodiment 3
[0100] Embodiment 3 macromolecular substance determination
[0101] 3.1 Determination method Determination method of macromolecular substances in Reduning Injection—Tymosin α1 as reference substance
[0102] Precisely measure 1ml of Reduning injection, add mobile phase to dilute to 5ml, shake well, and use it as the test solution; take an appropriate amount of thymosin α1 standard substance, weigh it, dissolve and dilute with water to make a solution containing 0.05mg per 1ml As a reference solution. Precisely measure 10 μl each of the test solution and the reference solution and inject it into the liquid chromatograph, record the chromatogram, regard the peak that is prior to the peak time of thymosin α1 (molecular weight 3108) as a polymer substance, and calculate it by the area normalization method Its content, macromolecular substances shall not exceed 0.05%.
[0103] Chromatographic conditions and system suitability test chromatographic column: TSK G2000SWXL (300mm×7.8m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com