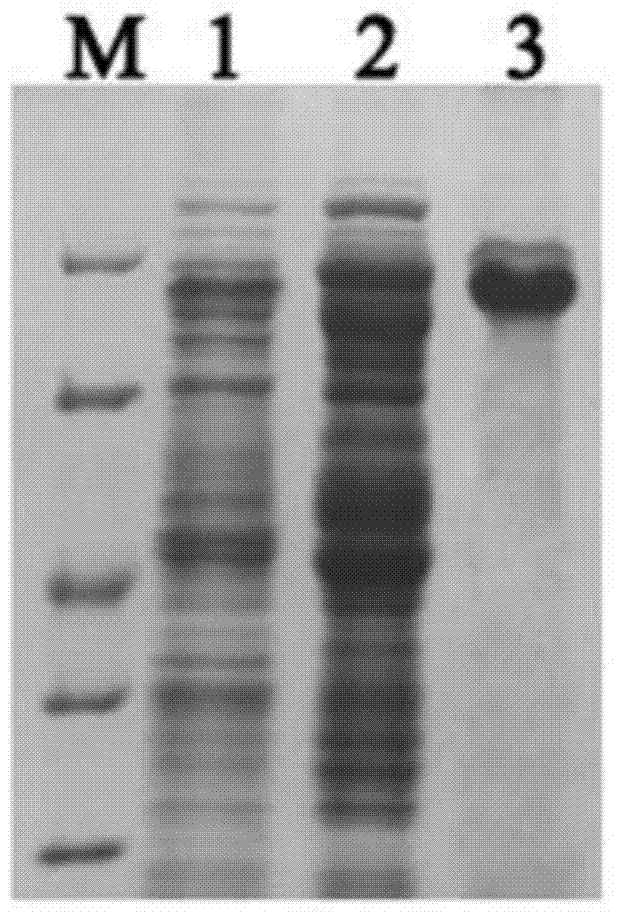
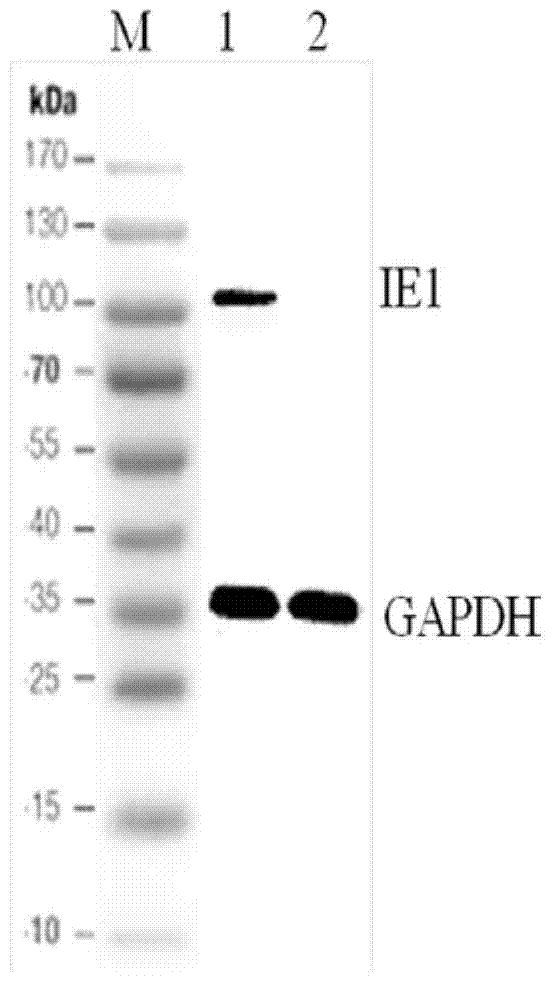
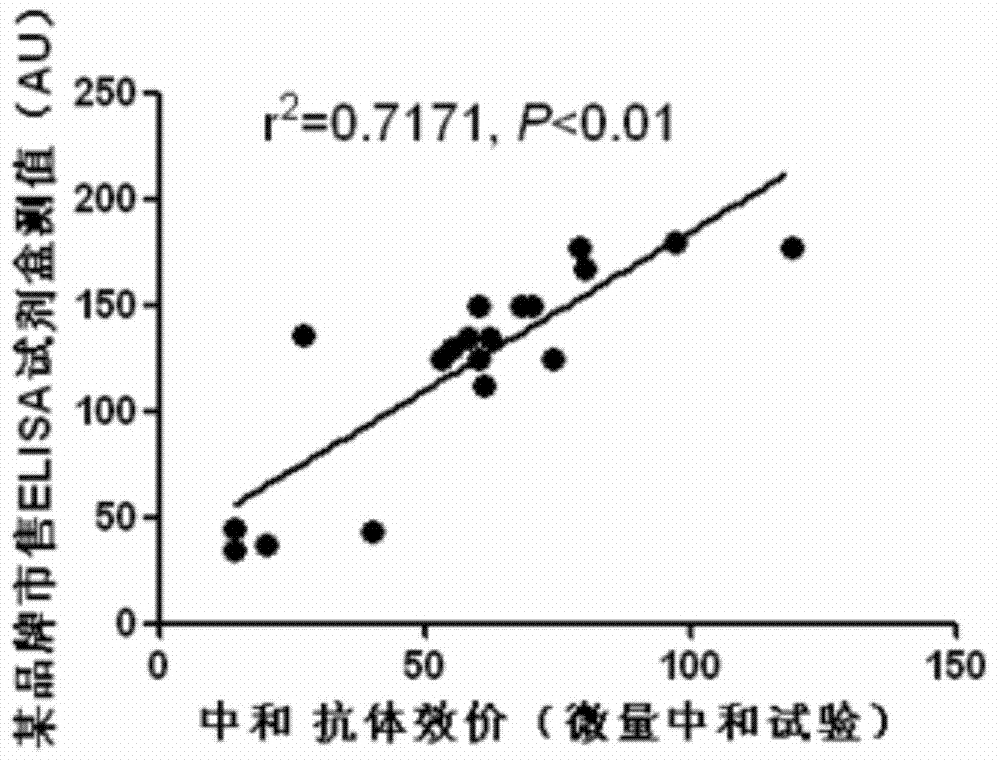
Preparation method of recombinant protein IE1-coated ELISA (Enzyme linked immune sorbent assay) reaction plate and assay kit for quantitatively detecting HCMV (human cytomegalovirus) neutralized antibody in human plasma
A recombinant protein and reaction plate technology, applied in the fields of genetic engineering technology, diagnostic reagents and blood product research and development, can solve the problems of pp150 protein without neutralizing virus, high titer plasma cannot be produced with specific intravenous gamma globulin preparations, etc. , to achieve the effect of easy screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Preparation of IE1 recombinant protein
[0026] 1. Preparation before reorganization
[0027] According to the HCMV gene sequence published in Genebank, the coding sequence of IE1 gene was determined, and PCR primers for amplifying exons 1 and 2 of IE1 gene were designed.
[0028] 2. RT-PCR acquisition of the target DNA fragment
[0029] RNA was extracted from HCMV AD169 strain virus culture as a template, cDNA was formed by reverse transcription, and the IE1 gene without intron was amplified by PCR.
[0030] 3. Insert the recombinant DNA fragment into the expression vector
[0031] The pET32a vector and the target DNA fragment were subjected to double enzyme digestion, and the digested product was purified and ligated by T4 DNA ligase. The ligated product was transformed into Escherichia coli BL21 (DE3) competent, and spread on the LB plate containing Amp at 37°C to incubate overnight. , Pick a single colony on the plate the next day for colony PCR and sequencing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com