Ring opening method of aziridine compounds
An aziridine and compound technology, applied in the field of organic synthesis, can solve the problems of long reaction time, high catalyst price, low selectivity and the like, and achieve the effects of low price, high yield and high reaction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
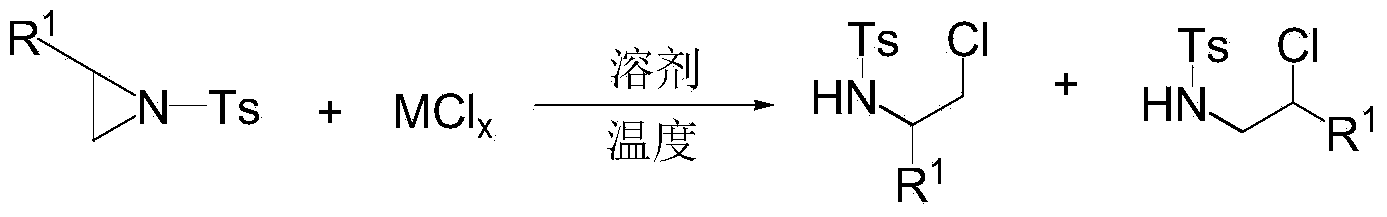
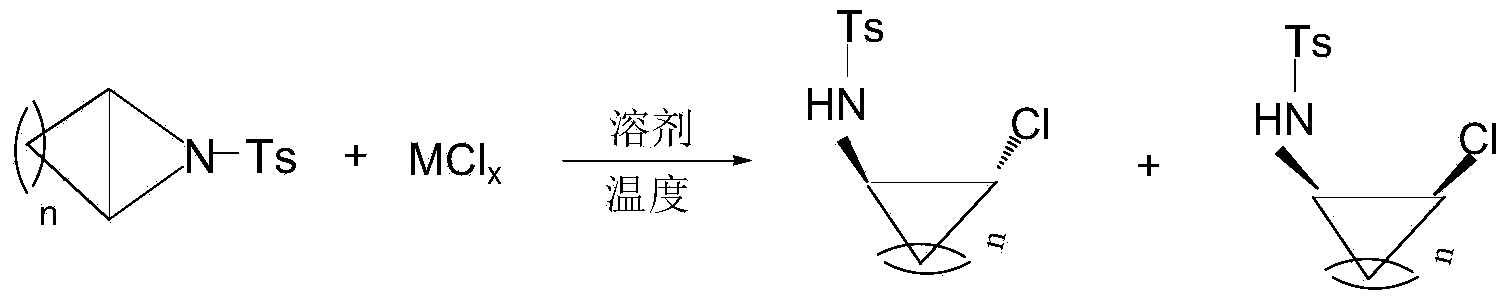
[0041] In a 50 mL round bottom flask, 1 mmol of the aziridine compound of the structural formula 1a in Table 1, 0.4 mmol of ferric chloride, and 10 mL of dichloromethane were added, and the reaction was stirred at room temperature 25°C for 0.5 h. After the reaction was completed, the concentrated solvent reaction system was purified by silica gel column chromatography to obtain the ring-opened product 3a as a white solid (eluent: petroleum ether: ethyl acetate = 5:1).
[0042] The regioselectivity of the ring-opening product was very high, and only the product of the structural formula 3a in Table 1 was obtained. The structure of the product was confirmed by infrared spectroscopy and NMR characterization, and the results are listed in Table 1.
[0043] The reaction of table 1 aziridine compound 1a and ferric chloride
[0044]
[0045] 3a:R f =0.2(Petroleum ether / AcOEt=5 / 1v / v);White solid;Mp:95-96℃;IR(KBr,cm -1 )3265(NH),1334,1155(S=O); 1 H NMR (600MHz, CDCl 3 ):δ2.44(s,...
Embodiment 2
[0047] Add 0.2mmol of the aziridine compound of the structural formula 1b in Table 2, 0.24mmol of sodium chloride, and 2.5mL of dichloromethane into the test tube, and stir the reaction at 20°C for 0.2h. After the reaction was completed, the concentrated solvent reaction system was purified by silica gel column chromatography to obtain the ring-opened product 3b as a white solid (eluent: petroleum ether: ethyl acetate = 5:1).
[0048]The regioselectivity of the ring-opening product is very high, and only the product of the structural formula 3b in Table 2 was obtained. The product was characterized by infrared spectroscopy, nuclear magnetic resonance and high-resolution mass spectrometry, and the structure of the product was confirmed. The results are listed in Table 2.
[0049] The reaction of table 2 aziridine compound 1b and sodium chloride
[0050]
[0051] 3b:R f =0.2(Petroleum ether / AcOEt=5 / 1v / v);White solid;Mp115-117℃;IR(KBr,cm -1 )3283,3064,2924,2854,1595,1490,140...
Embodiment 3
[0053] In a 50 mL round bottom flask, 1 mmol of the aziridine compound of the structural formula 1c in Table 3, 1.1 mmol of potassium chloride, and 5 mL of dichloromethane were added, and the reaction was stirred at 25° C. for 0.1 h. After the reaction was completed, the concentrated solvent reaction system was purified by silica gel column chromatography to obtain the ring-opened product 3c as a white solid (eluent: petroleum ether: ethyl acetate = 5:1).
[0054] The regioselectivity of the ring-opening product is very high, and only the product with the structural formula 3c in Table 3 was obtained. The product was characterized by infrared spectroscopy, nuclear magnetic resonance and high-resolution mass spectrometry, and the structure of the product was confirmed. The results are listed in Table 3.
[0055] The reaction of table 3 aziridine compound 1c and potassium chloride
[0056]
[0057] 3c:R f =0.12(Petroleum ether / AcOEt=5 / 1v / v);White solid;Mp88-90℃;IR(KBr,cm -1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com